
Research Director: Dr. Akihiro Kusumi
(Professor, Graduate School of Science, Nagoya University)
Research Term: 1998-2003
The most basic unit of life is the cell, a very small compartment which replicates itself. The cell is full of membranes which support its life, replication, and interactions with other cells as well as its environment. The outermost membrane that encloses the cell as a whole is called the “plasma membrane. ”
All cellular membranes have a general structure comprising a bilayer of molecules having affinity to both fat and water (amphiphilic lipid molecules) stuck end to end with the fatty part in the center and a waterphilic part at the extreme outside surfaces. Because of the loose binding of the lipids, they undergo fast conformational changes within the membrane, thus preventing their “freezing”(solidification). All membranes, including the plasma membrane, actually act as a type of two-dimensional liquid.
About half of the proteins in a cell are either inserted in the membrane (mostly sticking out) or bound to the membrane surface. They float around in this ocean-like liquid, propelled by the random Brownian motion of the lipids and water. However, the cell regulates their distribution, rate of diffusion, multimerization, and assembly in the plasma membrane for their specific functioning, though the processes involved are not understood. Although the random molecular motions (fluidity) of the membrane are important to allow the proteins to move around, other mechanisms that regulate their motions and positions must be at work to organize the protein and lipid molecules in the plasma membrane.
Regarding this situation, Akihiro Kusumi has proposed that the functions of the plasma membrane are regulated by the cytoskeleton, upon part of which the plasma membrane rests. Not only does the cytoskeleton appear to give structure and a degree of solidity to the cell, but it also appears to interact with the plasma membrane while helping to guide proteins to very specific cites where they can perform their functions.
Regarding this situation, Akihiro Kusumi has proposed that the functions of the plasma membrane are regulated by the cytoskeleton, upon part of which the plasma membrane rests. Not only does the cytoskeleton appear to give structure and a degree of solidity to the cell, but it also appears to interact with the plasma membrane while helping to guide proteins to very specific cites where they can perform their functions.
Analyses have shown two main forms of motion: a very rapid, but localized, fluctuation at the speed of the random Brownian motion, and a somewhat slow hopping motion between what appear to be different specific regions, or zones. To help define what these regions actually are, a protein with an attached gold particle was held by optical tweezers and then pulled around the membrane. At certain locations there was more resistance than elsewhere. By this mapping method it was found that the regions appear to be defined by the membrane part of the cytoskeleton the membrane skeleton. Thus, the fast motion occurs within small structures defined by the membrane skeleton, and the slower jumping-type motion occurs as the protein jumps from one compartment to another, to be defined by the membrane-associated part of the cytoskeleton – the membrane skeleton.
Still, despite these significant new insights concerning the behavior of the plasma membrane, there are still many things that are not known. One of these is the signaling that occurs which actually directs the proteins to move to certain locations where they perform their functions. Another problem is understanding the energy that is actually driving this motion.
Outline of Research
The Kusumi Membrane Organizer project is dedicated to understanding the cytoskeletal organization of the plasma membrane and how its constituent proteins are assembled at their specific locations to perform the critical cellular functions.
Research Results
Membrane anchored-protein “picket” model: It has been found that the membrane phospholipids are also compartmentalized, like the proteins. Their diffusion rates are from 10 to 100-times slower than that in artificial bilayers because the transmembrane proteins anchored to the membrane skeleton tend to act as rows of pickets, which interfere with the motion. This explanation solves a long-standing puzzle about the slow diffusion rate in the plasma membrane.
Single molecule probe scanning optical force imaging microscope for viewing live cells: An ultra-sensitive, single-molecule optical force scanning probe microscope has been developed that can measure the interaction force between a single membrane molecule and a component of the membrane/cytoskeleton in live cells. These individual forces give direct insight into protein-cytoskeleton and protein-lipid interactions which have previously only been inferred through the other techniques described above. With this increased sensitivity, new theoretical considerations were required. A theoretical framework has been developed based on simple physical concepts to understand/predict the behavior of single membrane molecules and to quantitatively look at the energetics of the membrane organization. Predictions from theory imply a dynamic nature to protein-barrier interactions, as is seen experimentally.
Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization: By observing the dynamics of individual phospholipid molecules in the plasma membrane of developing hippocampal neurons in culture, it was found that their diffusion was blocked in the axonal initial segment (IS) membrane. The barrier is formed in neurons 7-10 days after birth through the accumulation of various transmembrane proteins. It is concluded that various membrane proteins anchored to the dense membrane skeleton function as rows of pickets, which block diffusional mixing of proteins between the somatodendritic domain and the axon in polarized neurons.
Single molecule imaging of GFP in living cells: Single green fluorescent protein (GFP) molecules were successfully imaged for the first time in living cells. Using this technique it was found that E-cadherin, a trans-membrane protein responsible for recognition and adhesion between cells, exists in oligomeric forms, mostly greater than dimers, on the free cell surface, suggesting that these greater oligomers are the basic building blocks for the two-dimensional cell adhesion structures (adherens junctions).
Single molecule imaging of H-Ras activation in living cells: The activation of H-Ras, a GTP-binding protein involved in the signaling pathways for cell proliferation and reorganization of the cytoskeleton, has been visualized at the level of individual molecules using a technique called single-molecule fluorescence resonance energy transfer. In the resting state, the major population of H-Ras diffuses rapidly, whereas, at the time of signaling after external stimuli, the diffusion rate was shown to drop dramatically, which would explain localized signaling for polarized reorganization of the cytoskeleton.
Engagement of a GPI-anchored protein creates signaling rafts from smaller, transient, lipid rafts: Although membrane domain/rafts are implicated in receptor-mediated signaling and protein trafficking, their sizes, stability, and functional mechanisms have been controversial. Our single particle tracking analysis demonstrates that, in resting cells, the rafts appear small and unstable, and the consensus now is that their sizes are smaller than the optical diffraction limit (250 nm). Upon stimulation, the raft-preferring receptors are clustered, inducing larger, stabilized rafts, probably by coalescing small, unstable rafts or cholesterol-glycosphingolipid complex in the receptor clusters, including those in the inner leaflet of the membrane, each containing perhaps one molecule of the downstream effector molecules.
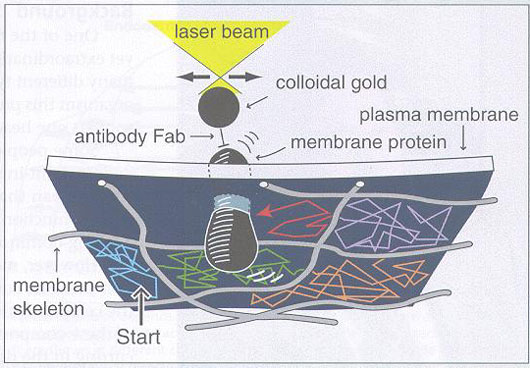
By observing and manipulating the movement of membrane receptors at the level of single molecules, membrane-skeletal organization of the plasma membrane is being elucidated.
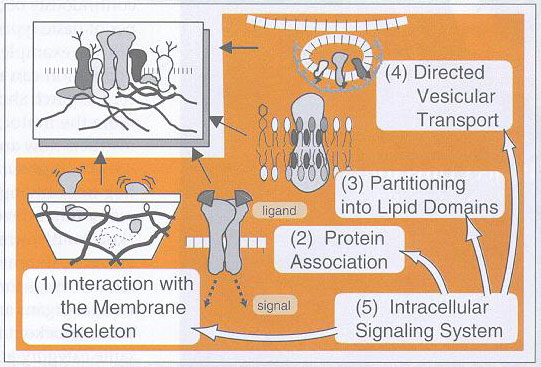
Five elementary processes for organization of the plasma membrane and formation of specialized membrane domains(working hypothesis)