
Research Director: Dr. Toshio Yanagida
(Professor, Faculty of Engeneering Science and Medical School, Osaka University)
Research Term: 1992~1997
One of the greatest mysteries of science is how such a large organism as the human being with billions of individual cells and their sub-components can move with smoothness and energy efficiency as a unified whole. The Yanagida Biomotron Project aimed to uncover the uniquely biological operations and structure of the biometron while also understanding how biological systems differ from those that are mechanical and human-made.
Research result
New technologies:
In order to understand how biomotrons operate, new technologies were developed to enable the detection of single biomolecules, single chemical reactions and conformational states of single protein molecules, and also to manipulate single molecules. Using a new microscope, the sliding of a fluorescently labeled motor protein(kenesin, found in nerve cells) along filaments called microtubles was visualized for the first time in real time. Also, a fluorescently labeled ATP analog was synthesized, and its hydrolysis reaction by motor proteins was visualized for the first time at a single molecular level. This microscope was also used to monitor the sliding movement of a DNA-related motor along DNA.
Imaging of single molecules:
The technology for imaging single molecules was extended to observe the structural changes within single protein molecules.
Manipulation of single protein molecules:
Technology to manipulate single molecules(such as a microneedle) as well as an optical-trap method were developed to measure the force produced by single motor proteins. These data showed that the mechanical reaction cycle occurs not once, but several times while a single ATP molecule is hydrolyzed.
ATP hydrolysis and mechanical work:
To directly determine how ATP hydrolysis is coupled with mechanical events, two technologies(single-molecule imaging anf a single motor force measurement) were combined so as to simultaneously measure the ATP hydrolysis reaction and force production, thus opening up the possibility to directly determine the coupling among the chemical reaction, conformational changes of motors and mechanical events.
Visualization of molecular interaction:
Various technologies were combined to construct an inter-molecular force microscope in order to visualize the interaction between molecules which form the biomotron, as well as its dynamic changes responding to chemical energy or physiological effectors. The technologies developed include micro-scanning probes of which the sensitivity is 100-times greater than that of AFM, a system which can control the thermal vibrations of the probe by laser radiation pressure, and the trapping and manipulation of a single protein molecule. This microscope will most likely become a powerful tool over wide fields of biology.
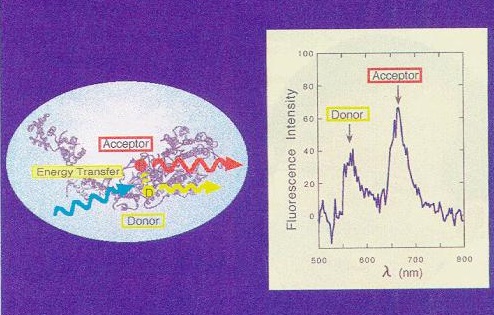
·Determination of conformational state for single protein molecules
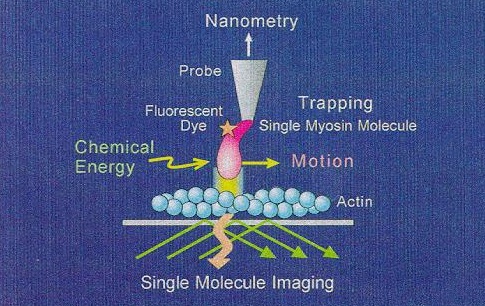
·Detection of inter-molecular force and chemical reaction in a single molecular level