Result
Last Update:Oct 10, 2014
Home > Result
Darwinian evolution of a translation-coupled RNA replication system in a cell-like compartment
Abstract
The ability to evolve is a key characteristic that distinguishes living things from non-living chemical compounds. The construction of an evolvable cell-like system entirely from non-living molecules has been a major challenge. In this study, we construct an evolvable artificial cell model from an assembly of biochemical molecules. The artificial cell model contains artificial genomic RNA that replicates through the translation of its encoded RNA replicase. We perform a long-term (600-generation) replication experiment using this system, in which mutations are spontaneously introduced into the RNA by replication error, and highly replicable mutants dominate the population according to Darwinian principles. During evolution, the genomic RNA gradually reinforces its interaction with the translated replicase, thereby acquiring competitiveness against selfish (parasitic) RNAs. This study provides the first experimental evidence that replicating systems can be developed through Darwinian evolution in a cell-like compartment, even in the presence of parasitic replicators.
Reference
http://www.jst.go.jp/pr/announce/20131003-2/index.html
In vitro evolution of α-hemolysin using a liposome display
Abstract
In vitro methods have enabled the rapid and efficient evolution of proteins and successful generation of novel and highly functional proteins. However, the available methods consider only globular proteins (e.g., antibodies and enzymes) and not membrane proteins despite the biological and pharmaceutical importance of the latter. In this study, we report the development of a method named liposome display that can evolve the properties of membrane proteins entirely In vitro. This method, which involves In vitro protein synthesis inside liposomes-cell-sized phospholipid vesicles-was applied to the pore-forming activity of α-hemolysin (AH), a membrane protein derived from Staphylococcus aureus. The obtained AH mutant possessed only two point mutations but exhibited a 30-fold increase in its pore-forming activity compared with the wild type. Given the ability to synthesize various membrane proteins and modify protein synthesis and functional screening conditions, this method will allow for the rapid and efficient evolution of a wide range of membrane proteins.
Reference
http://www.jst.go.jp/pr/announce/20131001-2/index.html
Coupling of the fusion and budding of giant phospholipid vesicles containing macromolecules
Abstract
Mechanisms that enabled primitive cell membranes to self-reproduce have been discussed based on the physicochemical properties of fatty acids; however, there must be a transition to modern cell membranes composed of phospholipids [Budin I, Szostak JW (2011) Proc Natl Acad Sci USA 108:5249–5254]. Thus, a growth-division mechanism of membranes that does not depend on the chemical nature of amphiphilic molecules must have existed. Here, we show that giant unilamellar vesicles composed of phospholipids can undergo the coupled process of fusion and budding transformation, which mimics cell growth and division. After gaining excess membrane by electrofusion, giant vesicles spontaneously transform into the budded shape only when they contain macromolecules (polymers) inside their aqueous core. This process is a result of the vesicle maximizing the translational entropy of the encapsulated polymers (depletion volume effect). Because the cell is a lipid membrane bag containing highly concentrated biopolymers, this coupling process that is induced by physical and nonspecific interactions may have a general importance in the self-reproduction of the early cellular compartments.
Figures
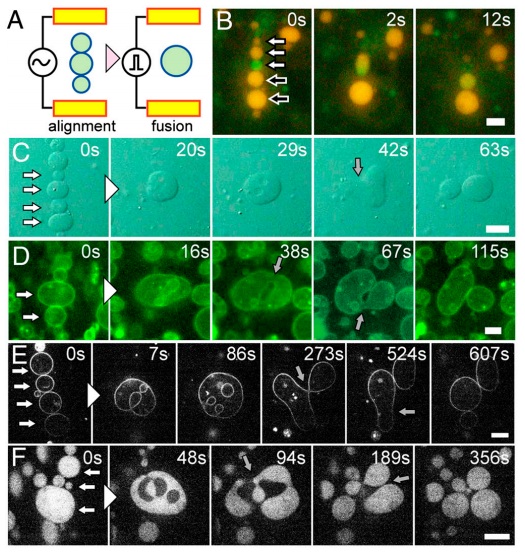
Fig. 1 Electrofusion and budding transformation of giant unilamellar vesicles (GUVs). (A) Schematic representation of the electrofusion experimental setup. (B) Sequential epifluorescence images of the electrofusion of GUVs containing GFP (green) and R-PE (orange) without polymer. White and black-filled arrows at time zero indicate vesicles to fuse together. (C–F) Sequential images of budding transformations of vesicles containing 3 mM PEG 6000. White-filled arrows at time zero indicate vesicles to fuse together. Gray-filled arrows indicate the neck formation before budding. (C) Bright-field images. (D) Epifluorescence images of the membrane marked with fluorescence lipids. (E) Confocal fluorescence images of the membrane marked with fluorescence lipids. (F) Confocal fluorescence images of the vesicles encapsulating FITC-BSA. (Scale bars: 10 μm.)
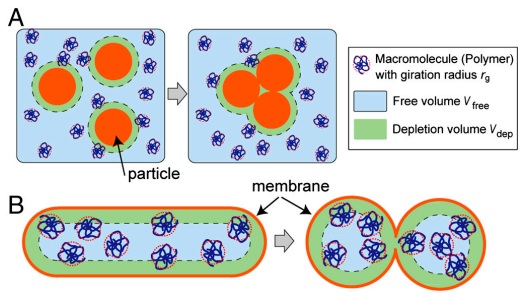
Fig. 2 Illustration of the depletion volume effect (not to scale). (A) Classical representation of the depletion volume effect. Larger particles in the polymer solution aggregate to reduce Vdep, in turn increasing Vfree. (B) In the system consisting of the GUV containing the polymer, Vfree increases when the curved area of the membrane increases, under the constraint of constant volume and surface area. Thickness of the polymer depletion volume is exaggerated to clarify the relative difference of Vfree.
Anticipated fruits of findings
We demonstrated the coupling of the fusion-tobudding transformation of phospholipid vesicles that mimics the growth and division of protocells using electrofusion and polymer-induced spontaneous vesicle shape transformation. After being elevated to the unstable state by fusion, the vesicle structure returns back to a state similar to the initial condition, which is thermodynamically (entropically) favored. Repetitive cycles were possible under the experimental condition because the constituents in the system remained identical after each cycle. In terms of the membrane physics, the emergence of spontaneous curvature due to the asymmetry of the solute across the membrane was predicted and demonstrated previously, but experimental verification with macromolecules was insufficiently explored. It is worth mentioning that the observed spontaneous transformation was induced by inert macromolecules at a weight concentration of approximately 5%. Because the modern cell contains macromolecules at a concentration of approximately 30%, this depletion interaction-based self-division might have had a general influence in the prebiotic-to-modern evolutionary pathway. Indeed, the stimulus required to gain excess membrane is not limited to electrofusion; it could be any energy fluctuation that may have existed in the prebiotic world and/or incorporation by the internal synthesis. Vesicle transformation due to the physical and nonspecific interactions presented in this work serves as an important model for protocell proliferation.
Links
Movies are available from the links below.
http://youtu.be/sY64KXAexIA
http://youtu.be/OCqvxpDAo-o
Reference
http://www.jst.go.jp/pr/announce/20120403/index.html
