Home > Research Contents|Main Research Results > Synthesis and Bioactivity of Natural Products, (+)-Chaetocin and Its Analogues
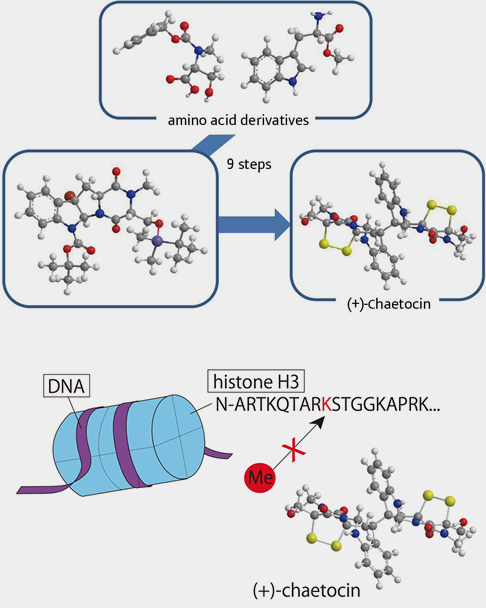
(+)-Chaetocin, which is isolated as a secondary metabolite from the fungus Chaetomium minutum, has been reported regarding its histone methyltransferase inhibition activity and cell-death-inducing activity, and is attracting much attention.
We succeeded in the first total synthesis of (+)-Chaetocin by establishing a short-step total synthesis from an amino acid as the starting material. Moreover, we applied the synthetic methodology for natural products to their analogues, and found that the optical isomer of a natural product had improved apoptosis-inducing activity compared to the natural isomer. We also clarified that its apoptosis-inducing activity was based on a different mechanism than that of present anti-cancer agents. Further structural expansion is being implemented at present, and application as a histone methyltransferase inhibitor or an anticancer agent is being investigated.
Iwasa E., Hamashima Y., Fujishiro S., Higuchi E., Ito A., Yoshida M., & Mikiko Sodeoka. Total Synthesis of (+)-Chaetocin and its Analogues: Their Histone Methyltransferase G9a Inhibitory Activity, J. Am. Chem. Soc. 2010, 132, 4078-4079,
Yuou Teng, Katsuya Iuchi, Eriko Iwasa, Shinya Fujishiro, Yoshitaka Hamashima, Kosuke Dodo, Mikiko Sodeoka. Unnatural enantiomer of chaetocin shows strong apoptosis-inducing activity through caspase-8/caspase-3 activation, Bioorg. & Med. Chem. Lett, 20, 5085-5088 (2010).








