Many cats develop chronic renal disorders as they age. As chronic renal disorders progress, the secretion of erythropoietin (EPO)(1), a hematopoietic factor produced in the kidneys, is decreased, which causes renal anemia(2). Veterinary medicine, until now, has only had an option of using erythropoietin (human EPO) derived from human amino acid sequence to treat severe renal anemia. However, since human EPO is a heterologous protein for cats, there has been an issue of adverse reactions, causing such as allergic reactions. Therefore, there has been a great demand for a renal anemia remedy, with a low level of adverse reactions for cats, that can lower the burden on both cats and their owners.
In the JST Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP), corporative development for an “anemia remedy produced through transgenic chicken technology” has been entrusted to Kaneka Corporation, based on the research results of Professor Shinji Iijima, currently of Aichi Institute of Technology (Nagoya University at the time of development) and others.
In this development, transgenic chicken(3) production technology was successfully used to develop feline (cat) erythropoietin (fEPO) with feline-derived amino acid sequences. A viral vector expressing cat EPO genes was injected into the embryo of fertilized chicken egg by micro injection(4), by which fEPO was produced within the egg albumin fraction. An established process in which Polyethylene glycol (PEG) modification(5) was conducted on fEPO collected and refined from chicken eggs lead to produce Pegylated fEPO with a longer efficacy period compared to unmodified fEPO (Fig.1). The Pegylated fEPO was tested on 60 cases in clinical trial, and its effectiveness and safety were evaluated (Fig.2). JST certified the results of the development related to this issue to be a success.
The developed Pegylated fEPO has little adverse reactions, therefore, serial administration to cats from the early stages in which symptoms of anemia occur is possible. Moreover, amelioration of symptoms such as loss of appetite and vitality due to anemia promises to lower the burden and improve the QOL of both the cat and its owner.
Kaneka will license out the developed outcome to domestic animal drug companies, and the companies assigned for the license will manufacture and sell the product.
A-STEP is a technology transfer support program whose aim is to put the research results by public research institutes into practical applications as important technology in the national economy, and thus to give some of their profit back to society. For detailed information, see https://www.jst.go.jp/tt/EN/univ-ip/a-step.html
<Glossary of terms>
(1) Erythropoietin (EPO)
This is a hormone that induces production of red blood cells. This is mainly produced in the kidneys. Transgenic erythropoietin formulations, such as epoetin alpha (product name Espo) and epoetin beta (product name Epotin), have been brought to the market as drugs for humans, and they are used as renal anemia remedies.
(2) Renal anemia
If renal function decreases due to renal disorder etc., the secretion of erythropoietin from the kidneys is reduced, and hematopoiesis is reduced. For this reason, if patients suffer chronic renal disease, they often tend to have anemia.
(3) Transgenic chickens
These are chickens to which foreign genes have been introduced. Methods of producing useful proteins, such as erythropoietin and antibodies using transgenic animals are gaining attention as animal factories. Among these, in particular, the production of eggs that store useful proteins laid by transgenic chickens is low in cost, and as the chicken can be raised quickly and proliferated in a short period of time, it can be used flexibly from high-mix low-volume production of proteins for medical use to the mass production of enzymes for industrial use. Special facilities that have received ministerial confirmation of containment measures, such as type 2 use, related to the Cartagena protocol, are established and used for the production of transgenic chickens used in this development.
(4) Micro injection
This is a method of injecting DNA within cells using an extremely fine glass tube. Generally, the glass tube is operated under a microscope, allowing the target substance to be injected within the cells one by one, which accomplishes a good expression efficiency. However, the method is not suitable to inject into a large number of cells at once, and dedicated equipment and a certain level of skill are required.
(5) Polyethylene glycol (PEG) modification
With the aim to improve pharmacokinetic properties of protein drug, the protein surface is modified with polyethylene glycol, a non-toxic and non-immunogenic macromolecule. PEG modification achieves to obtain the benefits of longer blood retention time, the water-solubilization of hydrophobic drugs, and a reduction of immunogenicity and antigenicity.
-
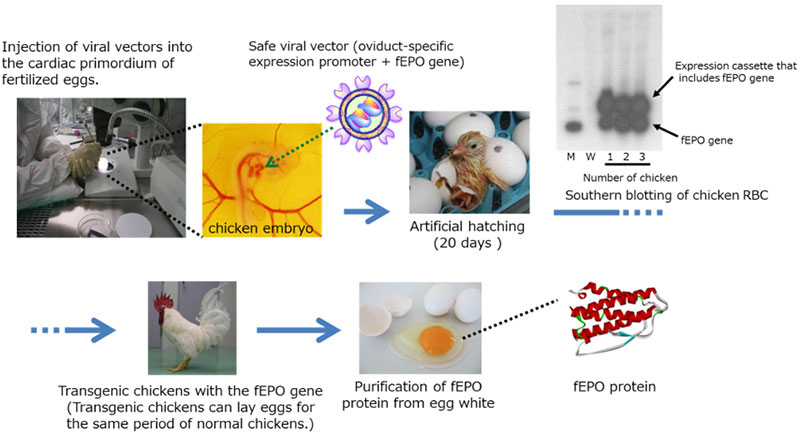
Fig.1 The production of fEPO by transgenic chicken ©Kaneka
-
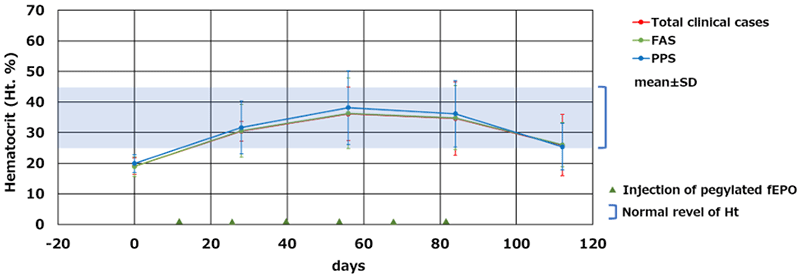
Fig.2 Changes in hematocrit levels after administration of fEPO Full analysis set (FAS) : All subjects which fEPO was administered one or more times. Per Protocol Set (PPS) : All subjects in the FAS which completed administration of cat EPO in treatment period. ©Kaneka
Program Information
- JST A-STEP (Adaptable and Seamless Technology Transfer Program through Target-driven R&D)
Contact
-
[About Research]
Hirokazu Sakamoto
Biotechnology Research Laboratories, Kaneka Corporation
TEL:+81-50-3133-7523
E-mail: Hirokazu.Sakamotokaneka.co.jp
-
[About Program]
Miho Okishiro
Department of Business Innovation Development, JST
E-mail: jitsuyokajst.go.jp
