Significance of Imitation and Action Understanding
Action understanding and imitation
Infants start to reach and grasp, or to imitate actions involving objects between six to nine months after birth. While infants under 12 months pay more attention to the movement of an action, those over 12 months can imitate the action regarding its goal or effect. It has also been indicated that even a three-month-old child can show understanding of an action regarding its goal after his/her own experience of object retrieval. These findings indicate that infants understand others’ actions based on their own action experience during development. Previous studies in neuroscience have also revealed that a specific brain region for action execution is utilized to understand others’ actions, which is called mirror neuron system (MNS). However, the relationship between MNS and different levels of action understanding remains unclear.
Ogawa and Inui (2008) used fMRI to investigate whether there is a common neural coding of action execution and recognition dependent on different action levels. Participants observed two movies showing the action of grasping a pen and putting it into one of two cups located on either side of a table. They then judged whether the first action was matched with the second action, regarding the following four aspects: 1) CUP: the cup in which the pen was placed (left or right cup); 2) GRASP: how the pen was grasped (thumb pointing upwards or downwards); 3) HAND: the hand used to grasp the pen (left or right hand); and 4) PATH: the rotating path of hand movement (clockwise or counterclockwise). Results showed that different brain regions were activated under each condition: medial prefrontal cortex (PFC) in CUP; left anterior intraparietal sulcus (AIP) in GRASP; bilateral superior parietal lobes (SPL) in HAND; and left premotor and primary motor areas in PATH. These regions correspond to areas suggested to be involved in motor control: PFC for action selection and monitoring; SPL for maintenance of hand states; AIP for control of grasping; and premotor areas for planning movement trajectories. Thus, the current study indicates that distinct brain regions are involved in observing different aspects of transitive actions, consistent with a hierarchically organized visuo-motor network of the observer’s own actions. This provides neuroscientific support for the developmental findings that infants’ own action experience facilitate the understanding of others’ actions.
Action recognition / developmental model of imitation and MNS
We employed multi-voxel pattern analysis (MVPA) on fMRI data to investigate the neural representation of observed actions in the human parietal and premotor cortex, which comprise the action observation network or the mirror neuron system for action recognition. Participants observed object-directed hand actions, in which the action and other properties were independently manipulated: action (grasp or touch), object (cup or bottle), perspective (1st or 3rd person), hand (right or left), and image size (large or small). We then used MVPA to determine whether each feature could be correctly decoded from regional activities. The early visual area showed significant above-chance classification accuracy, and was particularly high for perspective, hand, and size, consistent with pixel-wise dissimilarity of stimuli. In contrast, the highest action decoding accuracy was observed in the anterior intraparietal sulcus (AIPS) and the ventral premotor cortex (PMv). Moreover, the action decoder could be correctly generalized for images with high dissimilarity in the parietal and premotor region, but not in the visual area. These results indicate that the parietal and premotor regions encode observed actions independent of retinal variations, which may subserve our capacity for invariant action recognition of others. This study thus reveals a hierarchical visuo-motor network for action recognition from early visual to parietal and premotor areas.
Copying, tracing and reference frames
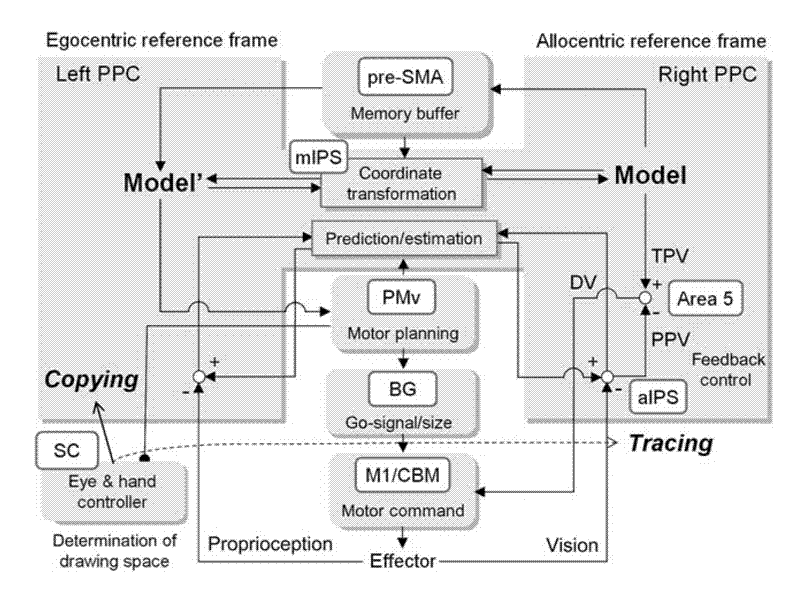
As noted above, construction as well as mutual transformation of egocentric and allocentric coordinates is critical for human development. The coordinate transformation is essential for figure drawing. This is particularly true for copying that requires reproducing an egocentric representation of the model trajectory relative to the end effector in a drawing space that is spatially separated from the model. During figure copying, infants often draw near the model or overlap the model, which is called closing-in phenomenon (CIP). CIP can be observed in adult patients with parietal damage (e.g. Alzheimer’s disease).
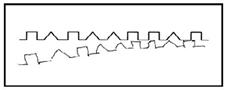
Fig. 1 An example of the closing-in phenomenon (CIP).
Williams syndrome (WS) is a rare neurodevelopmental disorder caused by hemizygous microdeletion of about 28 genes on chromosome 7q11.23. WS is characterized by low IQ and relatively unimpaired language abilities in spite of severely impaired visuospatial abilities. Previous WS studies have revealed structural and functional abnormalities in the parietal lobe, particularly the intraparietal sulcus [Nagai2006 in Japanese; Nagai2008 in Japanese]. Individuals with WS exhibit CIP on a figure coping task even after 10 years of age. These patients also make specific errors characterized by incorrect angles on a copying task, but not on a tracing task. Our study demonstrated that when the model was presented for a short time interval, WS patients could only perceive a severely limited number of the model’s features (Figure 5-6) [Nagai, 2008 in Japanese]. This deficit is evident when compared to that of healthy children 5 -6 years-old [Nagai2010]. In addition, two types of CIP emerged depending on the task: the approach type when it was necessary to match the model with the copy, and the overlap type when it was required to fill in and complete the copy [Nagai, 2008 in Japanese].
As compared to healthy children, WS patients have a strong CIP tendency, which does not correlate with age, IQ, and drawing abilities. This tendency arises irrespective of the way of matching with the model [Nagai2011, submitted]. It also arises when WS patients are required to fill in and complete a copy [Nagai2009 in Japanese]. These results indicate that WS patients have severe deficits in a part of visuospatial working memory as well as a disengagement of attention from a target figure. These functions should emerge in parallel during normal development. .
To investigate the brain areas both commonly and differentially involved in copying and tracing, we used fMRI to measure brain activity during copying and tracing with normal adults [Ogawa2009]. At the trial onset, a curved line was displayed as a model on either the upper or lower half of the screen, in a pseudo-randomized manner. During this period, the participant passively observed the model for 3, 4 or 5 s. A mouse cursor was then displayed onscreen with an auditory cue, and the participant started to reproduce model patterns by drawing. Only the medial part of the bilateral intraparietal sulcus (mIPS) showed significantly increased activation for copying versus tracing under both visual and memory guidance. The mIPS, therefore, appeared to be involved in coordinate transformation for copying, independent of visual or memory guidance. Our results indicate the involvement of the ventral premotor area (PMv) in motor planning of a drawing trajectory; the pre-supplementary motor area (pre-SMA) was involved in internally guided drawing based on short-term memory of the model.
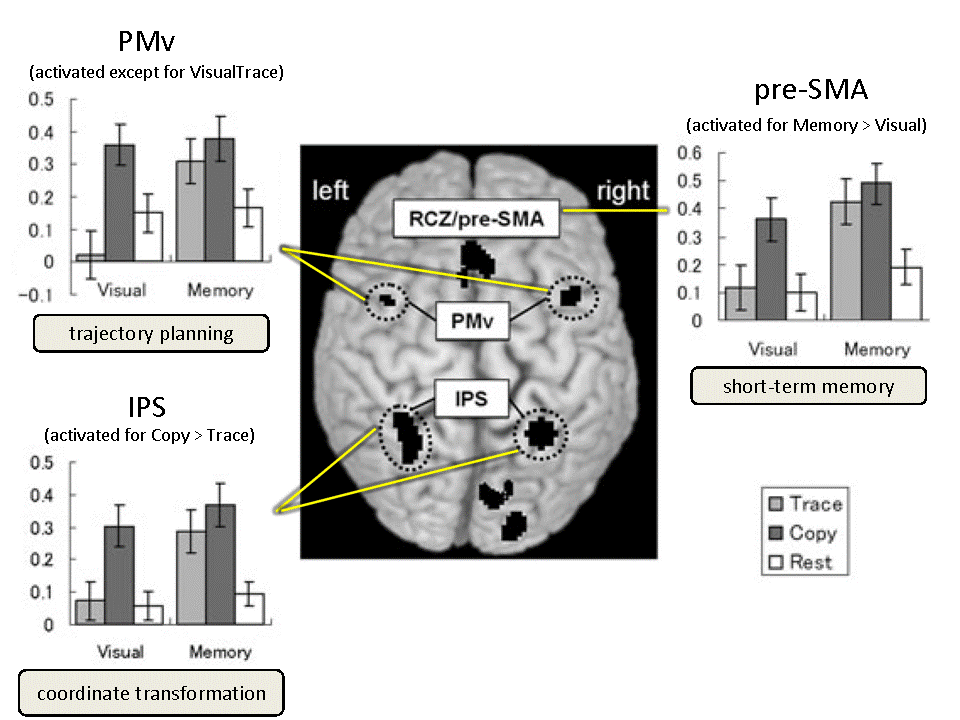
Fig. 2 Cortical activation during the copying and tracing tasks. [Ogawa2009]
To examine the reference frame used in the mIPS for visuo-motor transformation, we employed an fMRI MVPA for a delayed visually guided reaching task. A target was first presented either to the left or right of a fixation point. After a period of delay, participants moved a cursor to the position where the target had previously been displayed, using either a normal or a left-right reversed mouse. The activation patterns of normal sessions were first used to train the classifier to predict movement directions. The activity patterns of the reversed sessions were then used as decoder inputs to test whether predicted directions correspond to actual movement directions in either visual or body-centered coordinates. When the target was presented before actual movement, the predicted direction in the mIPS was congruent with the actual movement in the body-centered coordinates. This indicates that the mIPS uses body-centered coordinates to encode target positions or movement directions, which are crucial for visually guided movements. Our results also show that movement planning in the PMv is achieved with reference to a body-centered coordinate system.
A series of these studies lead us to speculate that the impaired copying abilities in WS patients may result from two factors [Inui2008 in Japanese]. One is their lower capacity for visuo-spatial working memory, which is required to temporarily maintain information about the external world and to use information to behave adequately. The other is the dysfunction of the conversion from allo- to ego-centered coordinates involving bilateral medial intraparietal sulci (MIP). Based on the findings by Ogawa & Inui (2007), we propose that egocentric and allocentric coordinates are dominantly represented in the left and right posterior parietal cortex, respectively. Furthermore, we indicate that inhibition of the reflex that coordinates the position of the hand with gaze direction (gaze position) may play an important role in the normal development of drawing behavior. We also suggest that the inhibitory pathway extends from the premotor cortex to the superior colliculus, and involves the generation of a drawing trajectory.