Japanese researchers have developed a simulation method to theoretically estimate the performance of heterogeneous catalyst by combining first-principles calculation (1) and kinetic calculation techniques. Up to now, simulation studies mainly focused on a single or limited number of reaction pathways, and it was difficult to estimate the efficiency of a catalytic reaction without experimental information.
Atsushi Ishikawa, Senior Researcher, Center for Green Research on Energy and Environmental Materials, National Institute for Materials Science (NIMS), performed computation of reaction kinetic information from first-principles calculations based on quantum mechanics, and developed methods and programs to carry out kinetic simulations without using experimental kinetic results. Then he applied the findings to the oxidative coupling of methane (OCM) reaction, which is an important process in the use of natural gas. He could successfully predict the yield of the products, such as ethane, without experimental information on the reaction kinetics. He also predicted changes in yield depending on the temperature and partial pressure, and the results reproduced faithfully the existing experimental results.
This research shows that the computer simulation enables the forecasting the conversion of reactant and the selectivity of products, even if experimental data are unavailable. The search for catalytic materials led by theory and calculation is expected to speed up. Furthermore, this method is highly versatile and can be applied not only to methane conversion catalysts but also to other catalyst systems such as for automobile exhaust gas purification, carbon dioxide reduction and hydrogen generation, and is expected to contribute to the realization of a carbon-free society.
The research was supported by JST's Strategic Basic Research Program, Precursory Research for Embryonic Science and Technology (PRESTO) Program.
(1) First-principles calculation
A theoretical calculation method for solving quantum mechanical equations without using empirical parameters, which is often applied to atomic, molecular, solid, and interface systems.
-
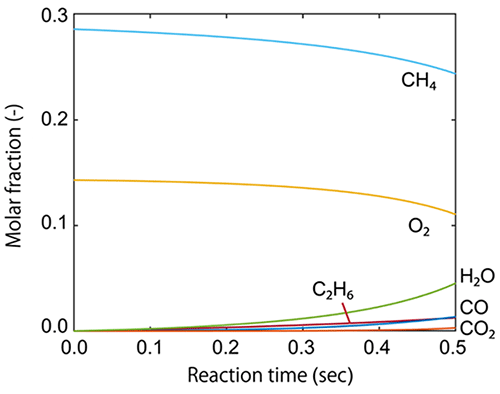
Figure 1. Mole fraction change Mole fraction along the reaction time (s) calculated by the reactor simulation. The inlet gas consisted of CH4, O2, and He (as inert gas). The total pressure was set to P = 1 bar, and the partial pressure ratio of CH4, O2, and He was set to 2:1:4. The volumetric flow rate was set to 1 mL/s, and the reaction temperature was 700 °C. The catalyst weight was 1 g. ©Atsushi Ishikawa
-
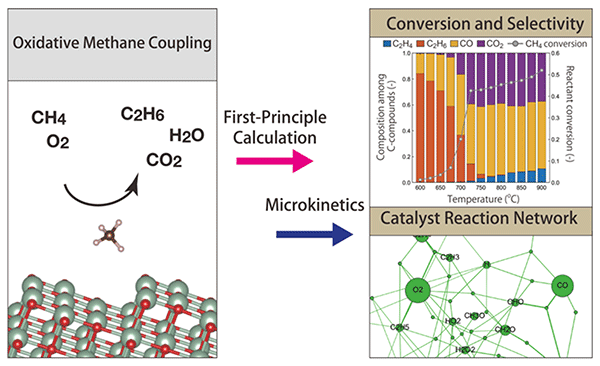
Figure 2. Concept of the study Graphical concept figure showing the combined approach of first-principle calculation and microkinetics. Catalytic activities such as conversion and selectivity are predicted. The catalytic reaction network is also obtained thus detailed analysis on the catalyst reaction is possible. ©Atsushi Ishikawa
Program Information
- JST PRESTO
- Research Area “Science and Creation of Innovative Catalysts”
- Research Theme “Development of combined approach of first-principle chemical kinetics simulations toward catalyst performance prediction and its application to methane conversion catalysts”
Journal Information
Atsushi Ishikawa and Yoshitaka Tateyama, “A First-Principles Microkinetics for Homogeneous–Heterogeneous Reactions: Application to Oxidative Coupling of Methane Catalyzed by Magnesium Oxide”, ACS Catalysis, Published online February 25, 2021, doi: 10.1021/acscatal.0c04104
Contact
-
[About Research]
Atsushi Ishikawa
Center for Green Research on Energy and Environmental Materials,
National Institute for Materials Science
TEL:+81-29-860-4610
E-mail: ISHIKAWA.Atsushinims.go.jp
-
[About Program]
Shimabayashi Yuko
Department of Strategic Basic Research, JST
E-mail: prestojst.go.jp
