Since mRNAs play a key role in protein synthesis in vivo, the use of mRNAs as medicines and for in vitro protein synthesis has been desired. In particular, mRNA therapeutics hold the potential for application to vaccine therapy(1) against coronaviruses and are being developed. However, the efficiency of protein production with mRNAs in the natural form is not sufficient enough for certain purposes, including application to mRNA therapeutics. Therefore, mRNA molecules allowing for efficient protein production have been required to be developed.
A ribosome(2) repeats the following three steps to synthesize a protein in vivo using an mRNA as a template (translation reaction): 1) Initiation step: A ribosome binds to an mRNA to form a translation initiation complex; 2) Elongation step: The ribosome moves on the mRNA and links amino acids to synthesize a protein; and 3) Termination step: The protein synthesis process concludes, and the ribosome is liberated. In the translation reaction cycle, the initiation step takes the longest time.
Collaborative research by a group of Nagoya University consisting of Professor Hiroshi Abe, Research Assistant Professor Naoko Abe, and graduate student Daisuke Kawaguchi with Yoshihiro Shimizu, a team leader at RIKEN, has succeeded in the development of modified messenger RNAs (mRNAs). The modified mRNA contains sulfur atoms in the place of oxygen atoms of phosphate moieties of natural mRNAs. It is capable of supporting protein synthesis at increased efficiency. They discovered that modified mRNAs accelerated the initiation step of the translation reactions and improved efficiency of protein synthesis by at least 20 times compared with that using natural-form mRNAs.
This method is expected to be used for large-scale synthesis of proteins as raw materials for the production of biomaterials. Moreover, the application of the results obtained in this study to eukaryotic translation systems enables the efficient production of mRNA therapeutics for protein replacement therapy(3) to contribute to medical treatments. Furthermore, there are virtually no previous reports on the molecular design of highly functional mRNAs; therefore, the successful design achieved in this study can guide a future direction of the molecular design of modified mRNAs.
This study was supported by the Strategic Basic Research Program CREST of the Japan Science and Technology Agency (JST).
(1)Vaccine therapy
A method of administering a protein antigen to individuals to elicit antibodies that can reduce the susceptibility to infectious diseases. In the case of an mRNA vaccine, an mRNA for in vivo expression of an antigen protein is administered, and then antibodies are produced against the expressed antigen protein.
(2)Ribosome
Multicomponent machinery providing a place where sequence information of an mRNA is read, and a protein is synthesized based on the sequence information while migrating on the mRNA. A ribosome is composed of ribosomal proteins and ribosomal RNAs.
(3)Protein replacement therapy
A treatment method that aims at improvement by supplementing protein from the outside when the deficiency of proteins (enzymes, etc.) is a cause of an illness.
-
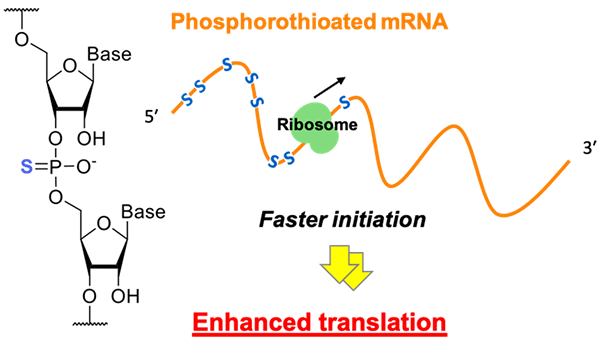
Figure. PS modification on mRNA enhances translation initiation Phosphorothioate modification of mRNA increases the rate of translation initiation and the molecular designs for a high translation efficiency were optimized. This result provides a useful molecular design guideline for improving the translation quantity by chemical modification of mRNA. ©Nagoya University
Program Information
- JST CREST
- Research Area “Large-scale genome synthesis and cell programming”
- Research Theme “Development of chemistry-based synthetic methodology for genome scale DNA”
Journal Information
Hiroshi Abe, Daisuke Kawaguchi, Ayumi Kodama, Naoko Abe, Kei Takebuchi, Fumitaka Hashiya, Fumiaki Tomoike, Kousuke Nakamoto, Yasuaki Kimura and Yoshihiro Shimizu, “Phosphorothioate Modification of mRNA Accelerates Rate of Translation Initiation Providing More Efficient Protein Synthesis”, Angewandte Chemie International Edition.. Published online July 5, 2020, doi:10.1002/anie.202007111
Contact
-
[About Research]
Abe Hiroshi
Graduate School of Science, Nagoya University
TEL:+81-52-789-2490
E-mail: h-abechem.nagoya-u.ac.jp
[About Program]
Yasuda Mutsuko
Department of Strategic Basic Research, JST
E-mail: crest
