

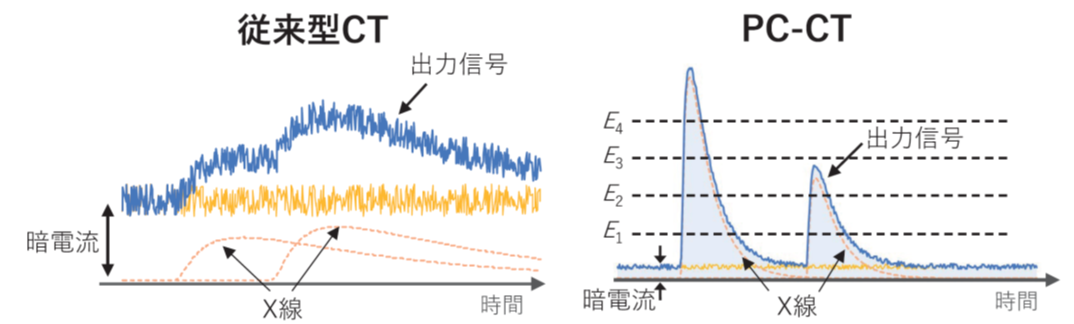
X線CTは、強力なX線照射を背景に、人体など被写体の影絵を取る技術です。現行CTの多くは セラミックシンチレータ(GOS:時定数 3μs)を光センサー(フォトダイオード)で読み出す構成であり、光センサー自体には増幅機能がありません。そのため、個々のX線パルスは微弱すぎて読み出すことができず、1mm角あたり 100Mcps にも及ぶ膨大なX線を照射することで、初めて積算値として電流を読み出すことが可能です。この状況では、(1) 1回の撮影でも被ばく量が大きくなる (10mSv程度。年間の自然放射線による被ばく線量の 5倍程度) (2) X線画像が色情報を失う、という2つの欠点が回避できません。
本グループが開発する「フォトン・カウンティングCT(PC-CT)」は、これらの問題を一挙に解決することが可能です。まず、X線の検出部には高速セラミックス・シンチレータ(YGAG: 時定数0.07μs)とSi-PM光センサー(増幅率 100万倍)を組み合わせます。これにより、1つ1つのX線信号をパルスとして読みだすことができ、エネルギー毎に色分けした「多色CT画像」を得ることができます。それぞれの物質は固有の色(吸収端)を持つため、これを強調することで造影剤や薬物など、必要な情報だけを抽出して画像化することが可能となります。さらに、PC-CT では閾値を設定することでノイズの寄与を効率よく取り除くことができるため、現行CTに比べて大幅な低被ばく化が期待できます
新開発のYGAGシンチレータ(プロテリアル社製)と 64ch のSi-PM アレイ(浜松ホトニクス社製)を用いた高速PC-CTシステムを立ち上げ、従来CTの 1/100 程度 (~ 1Mcps/mm2)の超低線量で画像を取得しました。PC-CTでは1つ1つのX線パルスが明確に識別でき、また断層画像でも水・アルコールが明確に識別できていることが分かります。データの読み出しに用いた高速アナログLSIは我々のグループで自作し、6色のエネルギー弁別による色付けを行うことができます。

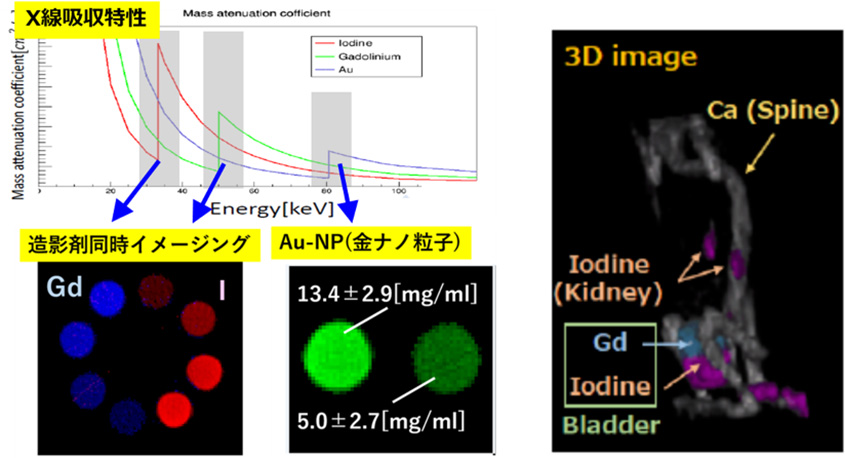
多色CT画像を用いて、代表的な造影剤である ヨウ素(I)、ガドリニウム(Gd)、さらには薬剤キャリアとしてもちいられる金ナノ粒子 (AuNP) の描出を試みました。ここで、ヨウ素は33keV, ガドリニウムは 50keV, 金は81keV に特徴的な吸収構造があることを利用し、そのエネルギー前後のCT画像を差し引いて物質弁別を行いました(Kエッジイメージングと呼ばれます)。また、2つの造影剤をマウスに注入し、ガドリニウム、ヨウ素の集積状況の違いを、生体マウスで同時にイメージングすることにも成功しました。
疾患モデルラットにGd-EOB-DTPA造影剤を2.0ml静脈注射し、健康なラットと脂肪肝ラットの肝臓に蓄積する造影剤を in-vivo/ex-vivo各条件下で多色CTイメージングし、その差異を定量的に調べました。健康なラットでは造影剤によるCT値の増加が顕著に確認できますが(青vs黒)、脂肪肝ラットではCT値の変化がほとんど見られません(緑vs赤)。これは、Gd-EOB-DPTAが正常な肝細胞のみに取り込まれる性質を反映していると考えられます。多色CT撮影により、肝臓にとりこまれた Gd-EOB-DPTA の濃度推定を行うことができ、健康なマウスでは 1.2mg/mL, 、脂肪肝マウスでは 0.2mg/mL 程度と、約6倍の開きがあることが分かりました。

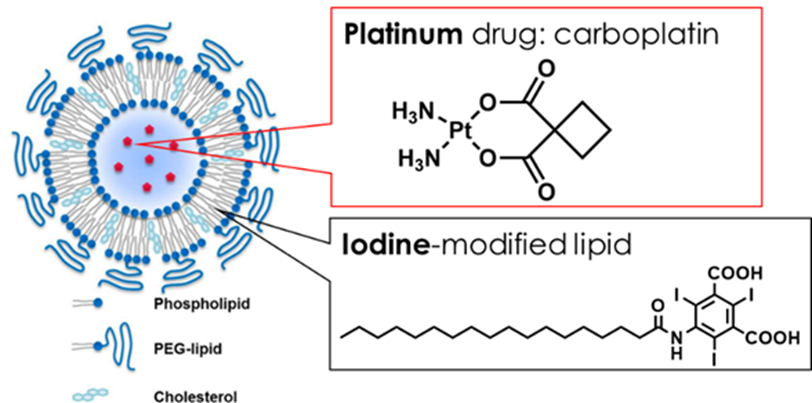
適量のお薬を、狙ったタイミングで患部に届ける薬物伝達システム(ドラッグデリバリーシステム:DDS)の開発は、世界中で急務の課題です。しかしながら、薬物が届く前にカプセルから漏洩する、あるいは届いてもカプセルが破れない、などの技術的困難から、これまで上市されたDDS薬剤は数種類に過ぎません。我々はプラチナ系抗がん剤であるカルボプラチンを 0.7mg/mL 内包し、カプセルとなるリポソームにヨード造影剤を 4.2mg/mL 修飾した新しい薬剤の合成に初めて成功しました。多色CTでは薬物本体(Pt)、カプセル(ヨード)それぞれの識別が可能なため、究極的には内包する薬物とカプセルの挙動を色分けして描画することができます。


X-ray CT is a technology that takes shadow images of subjects such as the human body using powerful X-ray irradiation. Most current CT scanners use ceramic scintillators (GOS: time constant 3μs) read out by optical sensors (photodiodes), which do not have amplification functions. Therefore, individual X-ray pulses are too weak to be read, and it is only possible to read the integrated value as a current by irradiating an enormous amount of X-rays, up to 100 Mcps per square millimeter. Under these circumstances, (1) the radiation exposure per scan becomes large (around 10 mSv, about five times the annual natural radiation exposure) and (2) the X-ray images lose color information, avoiding these two drawbacks is impossible.
The "Photon-Counting CT (PC-CT)" developed by our group can solve these problems simultaneously. First, the detection part of the X-rays combines a high-speed ceramic scintillator (YGAG: time constant 0.07μs) and an Si-PM optical sensor (amplification rate 1,000,000 times). This allows each X-ray signal to be read as a pulse, obtaining "multicolor CT images" color-coded by energy. Each material has a unique color (absorption edge), and by emphasizing this, it is possible to extract and image only the necessary information, such as contrast agents and drugs. Furthermore, PC-CT can efficiently remove noise contributions by setting thresholds, significantly reducing radiation exposure compared to current CT.

We established a high-speed PC-CT system using the newly developed YGAG scintillator (produced by Proterial) and a 64-channel Si-PM array (produced by Hamamatsu Photonics), acquiring images at ultra-low doses of about 1/100 of conventional CT (~1 Mcps/mm²). In PC-CT, each X-ray pulse is clearly identifiable, and it is apparent that water and alcohol can be distinctly identified even in tomographic images. The high-speed analog LSI used for data readout was self-made by our group, allowing for six-color energy discrimination.

We attempted to depict typical contrast agents, such as iodine (I), gadolinium (Gd), and gold nanoparticles (AuNPs) used as drug carriers, using multicolor CT images. Here, we utilized the characteristic absorption structures of iodine at 33 keV, gadolinium at 50 keV, and gold at 81 keV, performing material discrimination by subtracting CT images before and after these energies (called K-edge imaging). We also successfully imaged the accumulation of gadolinium and iodine simultaneously in live mice by injecting two contrast agents.

We injected 2.0 ml of Gd-EOB-DTPA contrast agent into disease model rats via intravenous injection and conducted multicolor CT imaging under both in-vivo and ex-vivo conditions to quantitatively investigate the differences in contrast agent accumulation in the livers of healthy and fatty liver rats. In healthy rats, a significant increase in CT values due to the contrast agent is confirmed (blue vs. black), while in fatty liver rats, almost no change in CT values is observed (green vs. red). This reflects the property of Gd-EOB-DPTA being taken up only by normal hepatocytes. Multicolor CT imaging allowed the estimation of the concentration of Gd-EOB-DPTA incorporated into the liver, revealing about six times the difference, with approximately 1.2 mg/mL in healthy mice and 0.2 mg/mL in fatty liver mice.

The development of drug delivery systems (DDS) that deliver an appropriate amount of medication to the affected area at the right time is an urgent task worldwide. However, technical difficulties such as leakage from the capsule before the drug reaches the target or failure of the capsule to rupture upon arrival mean that only a few DDS drugs have been marketed so far. We succeeded for the first time in synthesizing a new drug that encapsulates 0.7 mg/mL of the platinum-based anticancer drug carboplatin and modifies the capsule with 4.2 mg/mL of iodine contrast agent in liposomes. Multicolor CT allows for the identification of both the drug itself (Pt) and the capsule (iodine), enabling the ultimate visualization of the behavior of the encapsulated drug and the capsule.

One of the new possibilities using photon-counting CT is the evaluation experiment for advancing particle beam therapy. Typically, in particle beam therapy, images are acquired using X-ray CT during treatment planning, from which the irradiation field and dose distribution are calculated. However, the information obtained from CT involves interactions between matter and X-rays (mainly photoelectric absorption), with no physical causality to energy deposition by particle beams (Coulomb interaction). Usually, there is an error of about 3-5% when estimating the electron density in the body from CT values obtained by X-rays, which is a major factor limiting treatment accuracy. Therefore, this study aims to achieve high-precision estimation of electron density by combining multicolor CT images. Using a phantom with known electron density for calibration, we have succeeded in high-precision estimation of 2.7%.

We are exploring techniques using machine learning and sparse modeling to enhance CT image sharpness. For example, using our Sparse View CT technology, the number of imaging projections can be reduced to about 1/5, simultaneously reducing image noise by 34%. This is equivalent to reducing the CT scan time and lowering the dose. Moreover, it means that imaging with higher temporal resolution and visualization of lower concentration drugs (such as contrast agents) is possible.

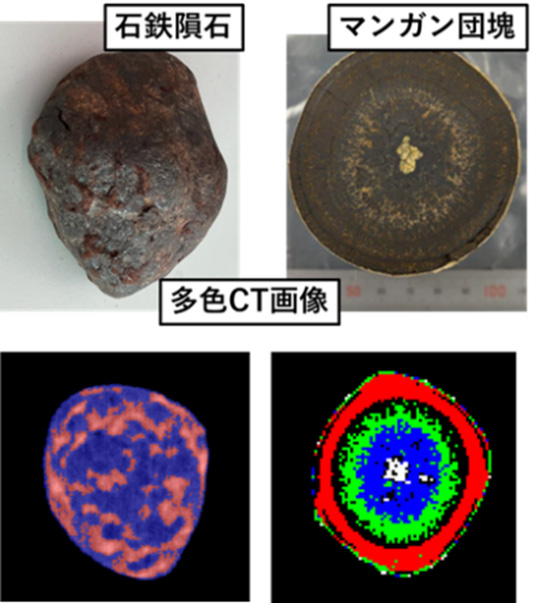
A new application of multicolor spectral CT is the non-destructive inspection of mineral resources (e.g., manganese nodules). Unlike current CT, which lacks energy information, the major advantage is the simple and easy identification of elements within minerals. By using photon-counting, the differences in X-ray absorption for each energy are clarified, and the spatial distribution of elements is clearly depicted. Furthermore, it is possible to obtain the internal composition of valuable geophysical samples, such as pallasites, without destruction and with high image quality.

最終更新日:2024年7月1日
Last Update: 1 July 2024