

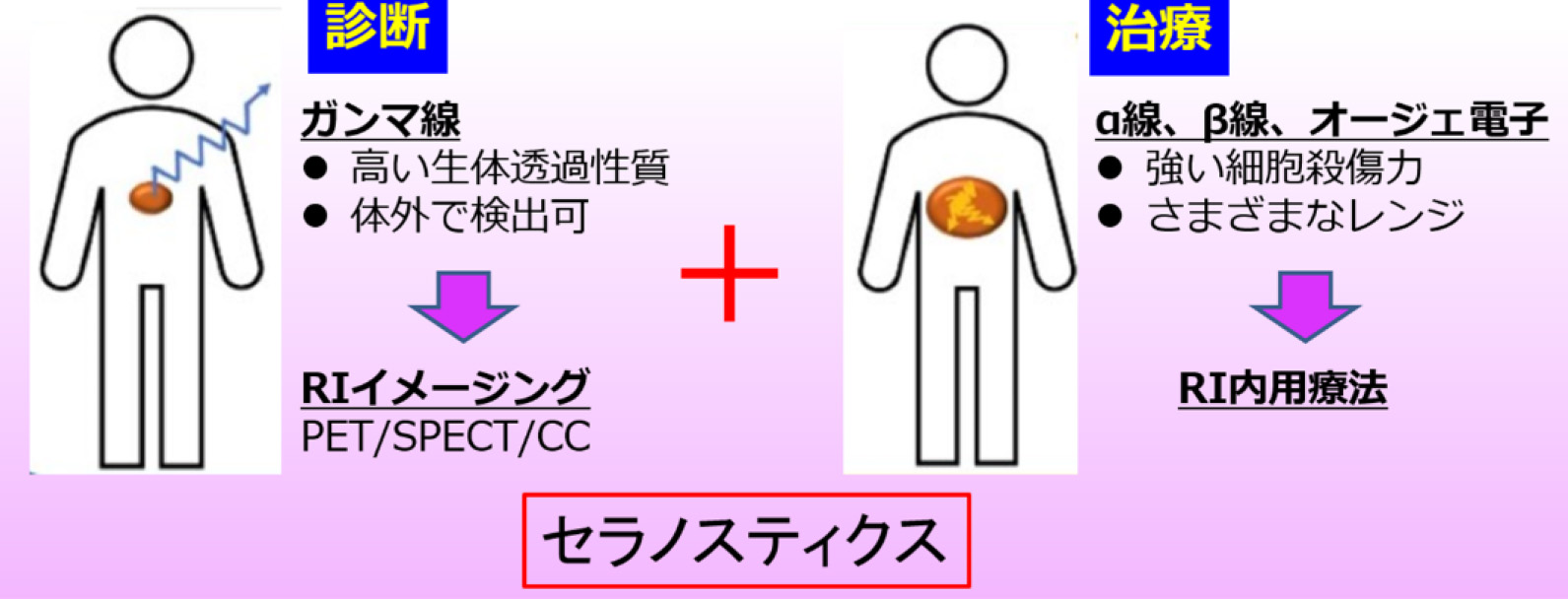
核医学診断とは、放射性同位元素を特定の疾患部やがん病巣に集中させ、そこから生ずるX線やガンマ線をプローブとして病巣を画像化する技術です。とくにSPECT(単一光子断層撮影)やPET(陽電子断層撮影)を用いた画像化が主流であり、がんの活性度など機能画像を得ることができる点が長所です。つまり、形態画像を取得するCTとは相補的な可視化技術と言えます。しかしながら、通常SPECTで可視化できるガンマ線は300keV程度以下に限られ、またPETでは原理上 511keV以外のガンマ線は画像化できません。そのため、核医学診断で用いられる放射性薬剤の数は、非常に限られています。
 本研究では、核医学診断の新たな地平線を広げるため、新しい可視化ツールである独自のコンプトンカメラを開発し、数十keV から数MeVに渡る広帯域のX線ガンマ線イメージングを1台で可能としました。さらに、コンプトンカメラを用いれば、診断のみならず、治療中に生ずる様々なX線・ガンマ線を「その場で」可視化することができ、核医学治療のモニタとしても利用することができます。現在、世界中で診断・治療を一体化した新しい医療技術「セラノスティクス」が注目を集めています。コンプトンカメラは、セラノスティクスの推進にも大きな貢献が見込まれます。特に、本研究では α線放出核種At-211 のキャリアである金ナノ粒子を放射化することで、これまで不可能であった薬物動態の長期可視化に挑戦します。
本研究では、核医学診断の新たな地平線を広げるため、新しい可視化ツールである独自のコンプトンカメラを開発し、数十keV から数MeVに渡る広帯域のX線ガンマ線イメージングを1台で可能としました。さらに、コンプトンカメラを用いれば、診断のみならず、治療中に生ずる様々なX線・ガンマ線を「その場で」可視化することができ、核医学治療のモニタとしても利用することができます。現在、世界中で診断・治療を一体化した新しい医療技術「セラノスティクス」が注目を集めています。コンプトンカメラは、セラノスティクスの推進にも大きな貢献が見込まれます。特に、本研究では α線放出核種At-211 のキャリアである金ナノ粒子を放射化することで、これまで不可能であった薬物動態の長期可視化に挑戦します。
さらに、金ナノ粒子はがんを体の外から狙い撃ちする放射線治療においても、治療効果を高める増感剤として注目を集めています。放射線治療には、X線、ガンマ線、陽子線や炭素線など様々な線種が用いられますが、それぞれにおける増感作用の比較や機序解明は、今後の課題です。本研究では、瞬間的に大強度のビームを照射するフラッシュ治療や、次世代治療である多核種ビームを用いた次世代粒子線治療についても、研究を進めています。
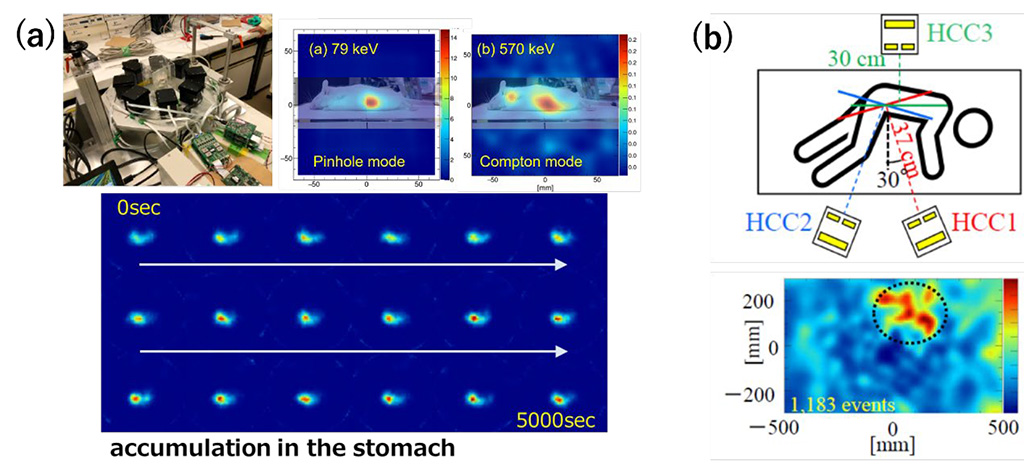
コンプトンカメラは散乱体・吸収体におけるエネルギー、反応位置からガンマ線の到来方向を推定する技術であり、視野が広く感度が高い反面、概ね200keV以下のガンマ線は散乱体で吸収され, イメージングできません。 そこで, 散乱体アレイに小孔を穿ち, 1台で30keVから数MeVをイメージング可能な「ハイブリッド・コンプトンカメラ(以下, Hybrid-CC)」を考案しました。Hybrid-CC を用いれば従来のPET/SPECTでは可視化が困難であったMeV領域までの様々な放射性薬剤を同時に可視化できます。一例として、α線治療薬であるAt-211から生ずる79keV/570keV のX線ガンマ線を用いたマウスの3次元イメージング結果を(a) に示します。また、阪大病院において骨転移ガンの治療薬である Ra-223を投与した患者さんのご協力をいただき、臨床イメージングを行った結果を (b)に示します。Ra-223は様々なガンマ線を放出するが、ここでは 80keVのX線をイメージングしました。コンプトンカメラは小型・コンパクトでありながら、10分程度の撮影で全身の薬剤分布を可視化することができます。
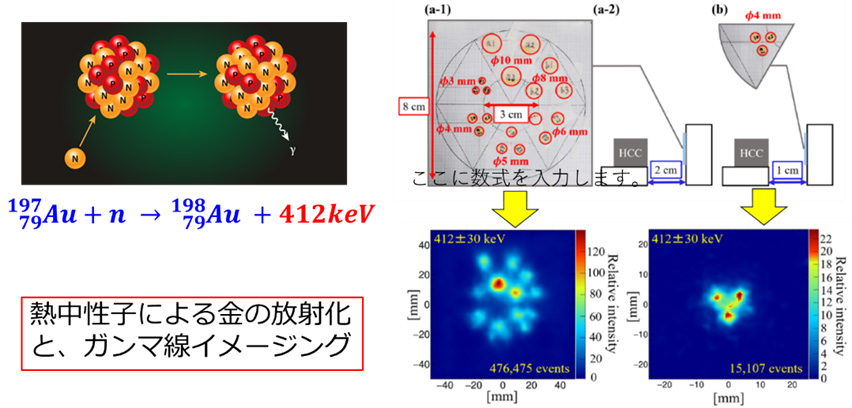
宇宙の元素合成をヒントに、標識が一切不要な「放射化イメージング法」を新たに創出しました。とくに抗がん剤や薬物キャリアで重要な金(原子番号79)やプラチナ(原子番号78)など重い元素は、中性子星合体など爆発的「中性子捕獲」で一気に作られたと示唆され、これを薬物動態イメージングに応用できます。本研究では、まず様々なサイズの金板を熱中性子で軽く放射化し、同位体198Au を生成しました。198Au は2.7日の半減期でβ線と412keV(キロ電子ボルト)のガンマ線を放出するため、Hybrid -CC を用いて短時間に可視化できることを示しました。
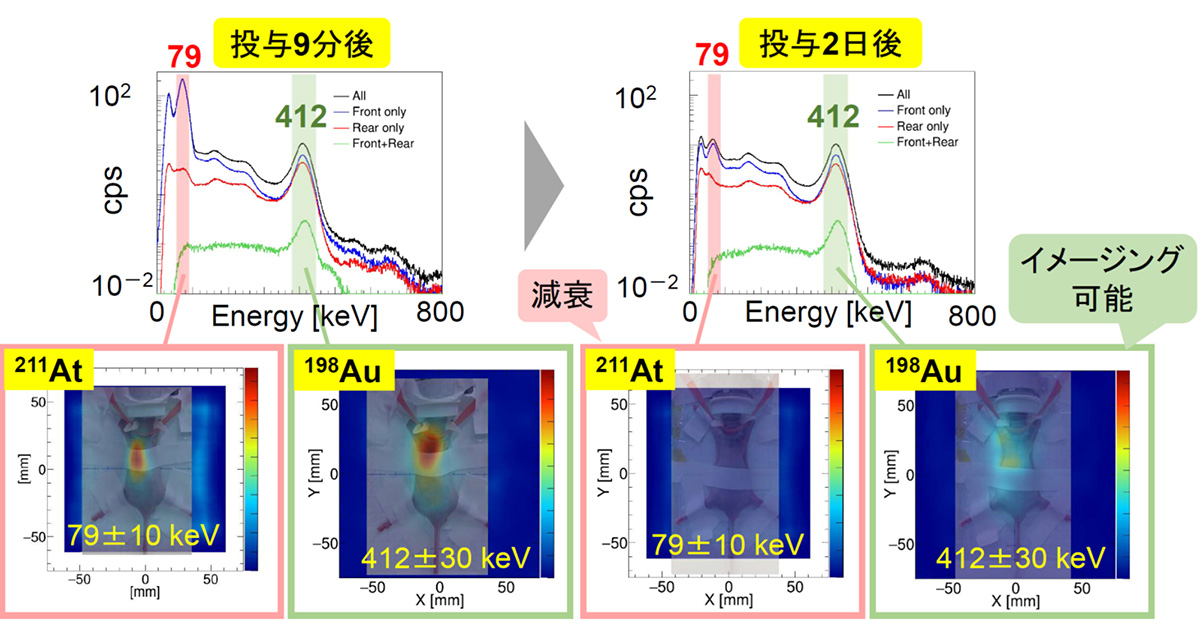
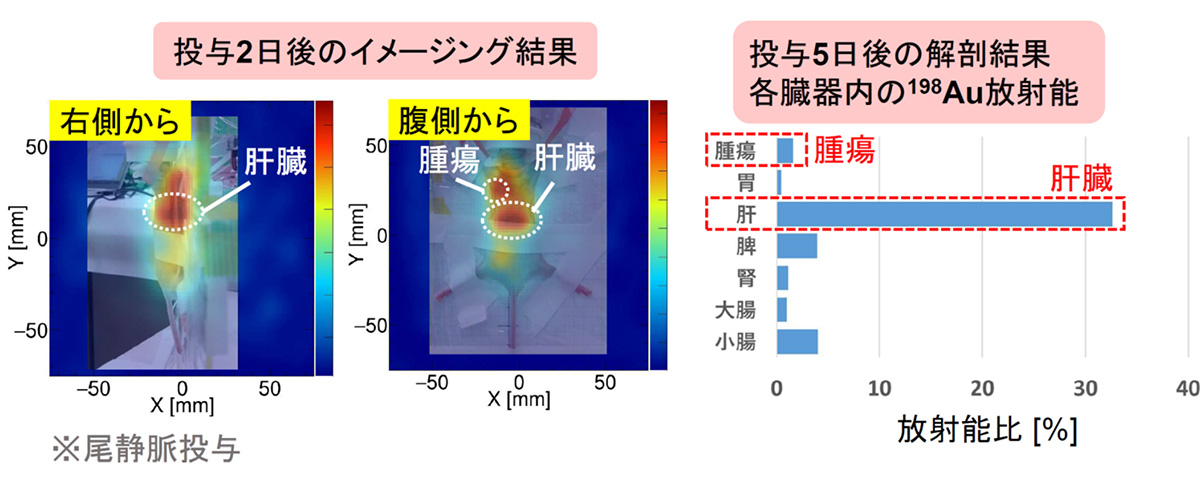
より本格的な医療応用に向け、放射化金ナノ粒子(Au-198)を安定かつ迅速に製造するプロトコルを確立し、核医学治療薬であるAt-211を担持させることにも成功しまし。ここで生成した[211At/198Au]AuNP-S-mPEGをマウスの尾静脈から投与し、α線治療薬であるAt-211から生ずる79keV X線および Au-198から生ずる412keVガンマ線の同時イメージングを試みました。投与9分後ではどちらも可視化できていますが、2日後には半減期7時間のAt-211の可視化が困難で、半減期が長い Au-198(412keV)だけが可視化できていることが分かります。ガンマ線画像では腫瘍と肝臓への集積が明確にみられ、解剖結果ともおおむね一致することが分かりました。
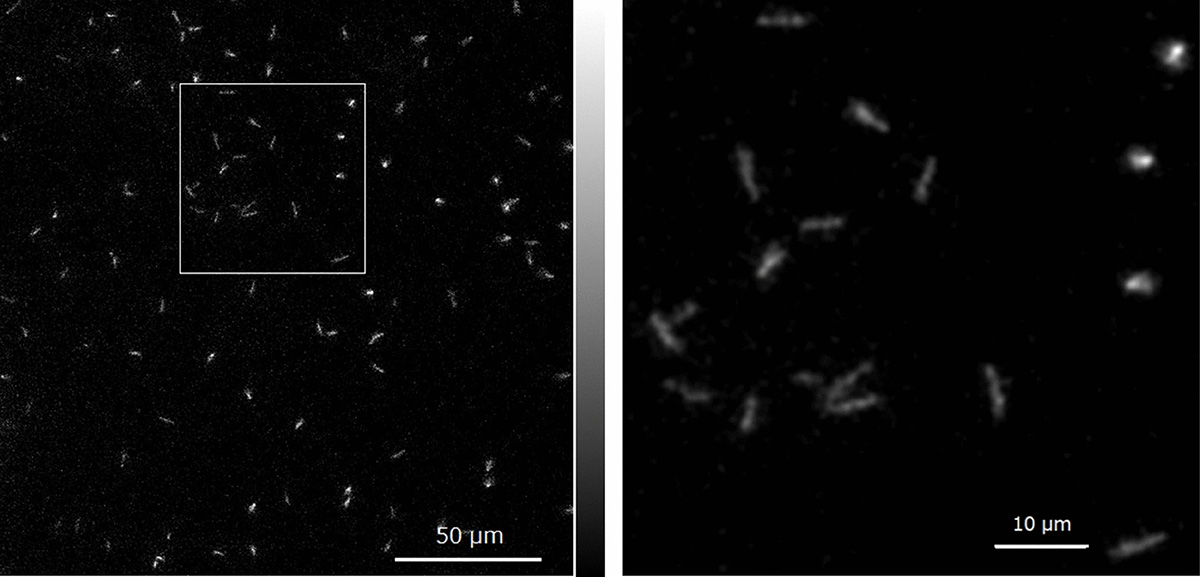
Hybrid-CCを用いたイメージングは臓器や体を対象としたマクロなサイズの可視化ですが、α線自身の飛程はわずか数十ミクロンです。がん細胞レベルの損傷を調べるには、飛跡にそった微視的なイメージングも、ミクロな観点から重要となります。そこで、X線顕微鏡とEM-CCDを組み合わせる新しい発想で、アルファ線の飛跡をリアルタイムかつ数ミクロンの超高解像度で可視化する、新しいカメラを開発しました。将来的には、がん細胞に取り込まれた At-211 等から生ずるアルファ線をリアルタイムで捉えることが可能となり、核医学分野への大きなブレークスルーが期待できます。
Nuclear medicine diagnostics involve concentrating radioactive isotopes in specific diseased areas or cancer lesions and using the resulting X-rays or gamma rays as probes to image the lesions. Imaging with SPECT (Single Photon Emission Computed Tomography) and PET (Positron Emission Tomography) is particularly prevalent, offering the advantage of functional imaging, such as cancer activity levels. In other words, this is a complementary visualization technique to CT, which captures morphological images. However, the gamma rays that can typically be visualized with SPECT are limited to around 300 keV or less, and PET, by principle, can only image gamma rays at 511 keV. Consequently, the number of radiopharmaceuticals used in nuclear medicine diagnostics is very limited.
 In this study, we developed a novel Compton camera, a new visualization tool, enabling wide-band X-ray and gamma-ray imaging ranging from several tens of keV to several MeV with a single device, to expand the horizons of nuclear medicine diagnostics. Moreover, the Compton camera can visualize various X-rays and gamma rays "on-site" not only for diagnostics but also during treatment, making it useful as a monitor for nuclear medicine therapy. Currently, a new medical technology integrating diagnosis and treatment, "theranostics," is attracting attention worldwide. The Compton camera is expected to make a significant contribution to the advancement of theranostics. Specifically, in this study, we are challenging the previously impossible long-term visualization of pharmacokinetics by activating gold nanoparticles, carriers of the alpha-emitting nuclide At-211.
In this study, we developed a novel Compton camera, a new visualization tool, enabling wide-band X-ray and gamma-ray imaging ranging from several tens of keV to several MeV with a single device, to expand the horizons of nuclear medicine diagnostics. Moreover, the Compton camera can visualize various X-rays and gamma rays "on-site" not only for diagnostics but also during treatment, making it useful as a monitor for nuclear medicine therapy. Currently, a new medical technology integrating diagnosis and treatment, "theranostics," is attracting attention worldwide. The Compton camera is expected to make a significant contribution to the advancement of theranostics. Specifically, in this study, we are challenging the previously impossible long-term visualization of pharmacokinetics by activating gold nanoparticles, carriers of the alpha-emitting nuclide At-211.
Furthermore, gold nanoparticles are also drawing attention as sensitizers to enhance therapeutic effects in radiation therapy that targets cancer from outside the body. Various radiation types such as X-rays, gamma rays, proton beams, and carbon beams are used in radiation therapy, but comparing their sensitizing effects and elucidating the mechanisms remain challenges for the future. This study also advances research on flash therapy, which irradiates with high-intensity beams instantaneously, and next-generation particle therapy using multi-nuclide beams, a next-generation treatment.
The Compton camera estimates the arrival direction of gamma rays based on the energy and reaction position in the scatterer and absorber, offering a wide field of view and high sensitivity. However, gamma rays generally below 200 keV are absorbed by the scatterer and cannot be imaged. Therefore, we conceived the "Hybrid Compton Camera (Hybrid-CC)" by drilling small holes in the scatterer array, allowing imaging from 30 keV to several MeV with a single device. Using Hybrid-CC enables simultaneous visualization of various radiopharmaceuticals in the MeV region, which was difficult with conventional PET/SPECT. For instance, (a) shows the three-dimensional imaging results of a mouse using 79 keV/570 keV X-rays and gamma rays from the alpha therapeutic agent At-211. Additionally, (b) shows clinical imaging results with the cooperation of a patient administered with the bone metastasis treatment agent Ra-223 at Osaka University Hospital. Ra-223 emits various gamma rays, but we imaged 80 keV X-rays here. Despite being compact and small, the Compton camera can visualize the distribution of the drug throughout the body with about 10 minutes of imaging.

Inspired by the nucleosynthesis in the universe, we created a new "activation imaging method" that requires no labeling. Heavy elements such as gold (atomic number 79) and platinum (atomic number 78), important in anticancer drugs and drug carriers, are suggested to be explosively synthesized in neutron capture during neutron star mergers. This can be applied to pharmacokinetic imaging. In this research, we lightly activated gold plates of various sizes with thermal neutrons, generating the isotope 198Au. 198Au emits beta rays and 412 keV gamma rays with a half-life of 2.7 days, demonstrating that it can be visualized in a short time using the Hybrid-CC.

For more comprehensive medical applications, we established a protocol to stably and quickly produce activated gold nanoparticles (Au-198) and successfully loaded them with the nuclear medicine therapeutic agent At-211. We attempted simultaneous imaging of 79 keV X-rays from At-211 and 412 keV gamma rays from Au-198 by administering the generated [211At/198Au]AuNP-S-mPEG through the tail vein of a mouse. At 9 minutes post-administration, both are visible, but after 2 days, At-211 with a half-life of 7 hours is challenging to visualize, while only Au-198 (412 keV) with a longer half-life is visible. The gamma-ray images clearly show accumulation in tumors and the liver, generally consistent with the dissection results.

Imaging with the Hybrid-CC is macroscopic, targeting organs and bodies, but the range of alpha rays themselves is only a few tens of microns. To examine damage at the cancer cell level, microscopic imaging along the trajectory of alpha rays is also important from a micro perspective. Therefore, we developed a new camera that visualizes the tracks of alpha rays in real-time with ultra-high resolution of a few microns by combining an X-ray microscope and an EM-CCD. In the future, it is expected to capture alpha rays from At-211 incorporated into cancer cells in real-time, representing a significant breakthrough in the field of nuclear medicine.

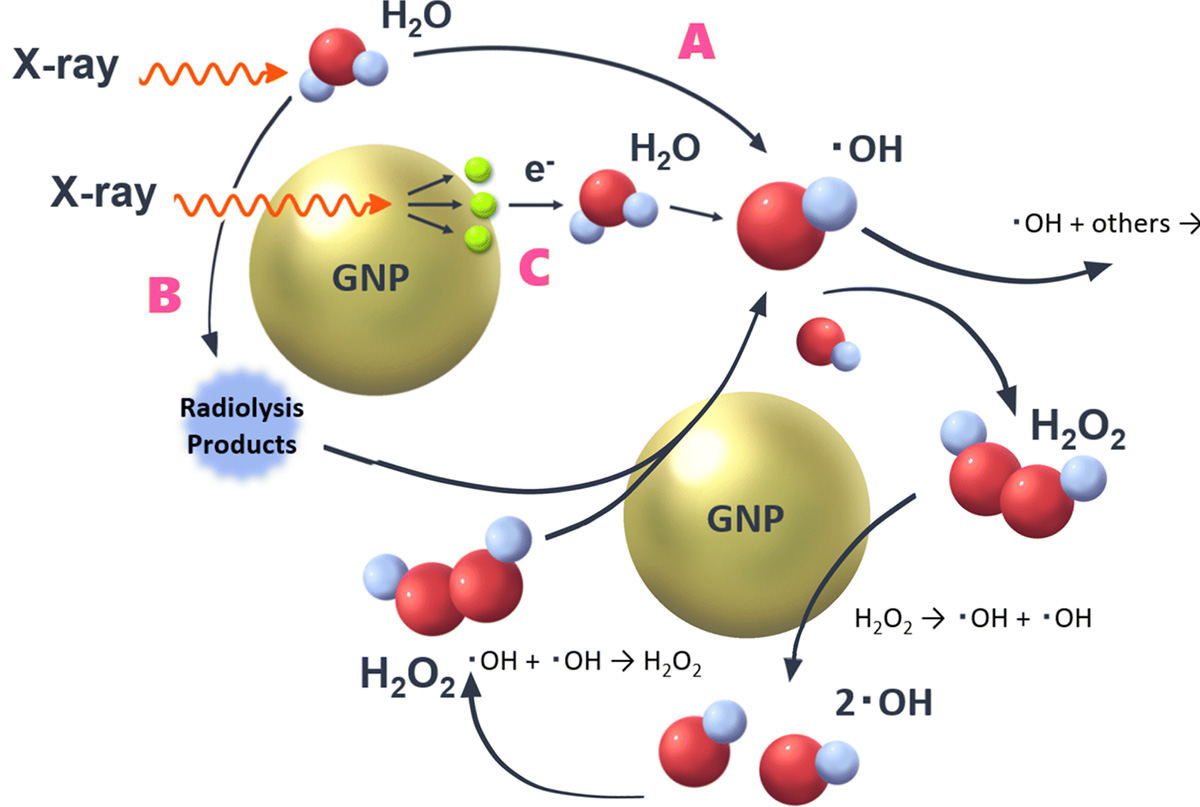
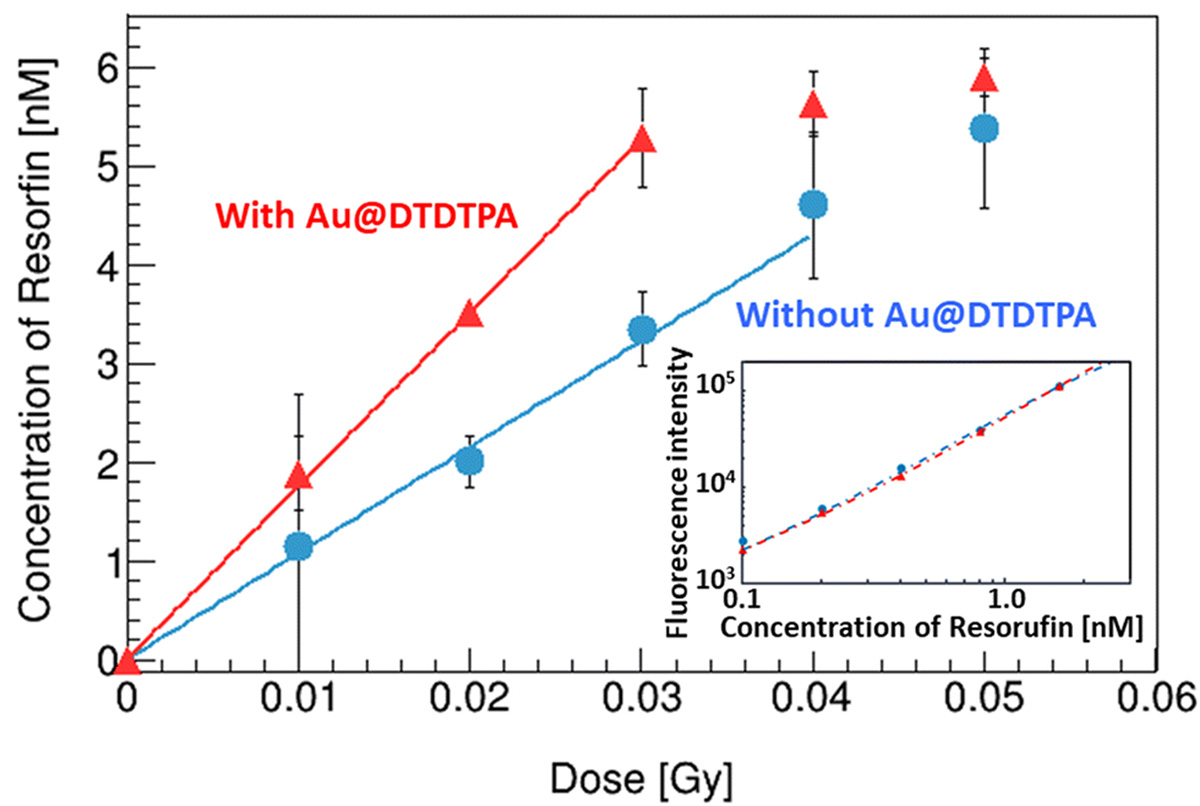
In radiation therapy, gold nanoparticles are known for their sensitizing effects, but the detailed mechanisms are not understood. We evaluated the yield of radiolysis products (OH radicals and hydrogen peroxide) generated in water when irradiated with X-rays, comparing with and without gold nanoparticles. The presence of gold nanoparticles is expected to cause surface reactions with the generated products, in addition to the usual water radiolysis. It was demonstrated that (1) in the presence of gold nanoparticles, hydrogen peroxide is decomposed, reducing its yield, and (2) new OH radicals are generated from the reaction H2O2 → *OH + *OH due to the decomposition of hydrogen peroxide. The decomposition of hydrogen peroxide by gold nanoparticles was 1.01±0.01 molecules/100 eV, and 2.02±0.02 OH radicals/100 eV were newly generated. This experimentally demonstrated for the first time that the increased OH radicals contribute to the sensitizing effect of gold nanoparticles.

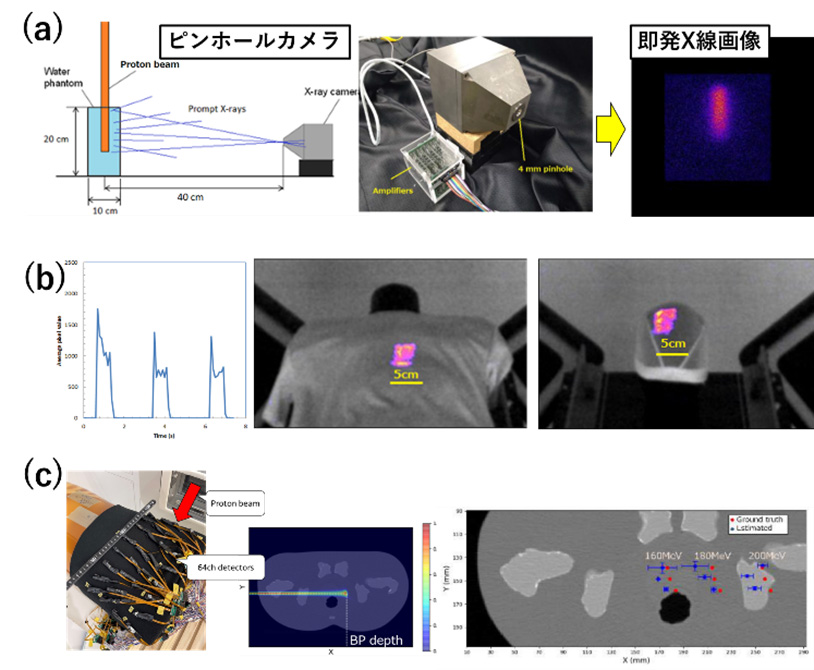
We are also challenging the visualization of invisible proton therapy beams, similar to gamma rays. For example, (a) verification of irradiation using prompt X-rays and gamma rays, (b) confirmation of irradiation position using radiation emission from polyester clothing, and (c) estimation of 3D irradiation dose distribution using scattered protons were conducted. In (a), bremsstrahlung from secondary electrons generated by proton beams is visualized. In (b), emission on the clothing surface can be seen, but depth distribution cannot be obtained. Although the emission of polyester is 10-20% compared to plastic scintillators, real-time video recording is possible using a CMOS camera. In (c), scattered protons follow Rutherford scattering, and the energy dependence is proportional to E^-1 like ionization interactions. This year, 64 small MPPC and CsI(Tl) sensors were arranged in an array on the surface of a human phantom, and dose distribution estimation was performed with a treatment beam assuming prostate cancer. Although an error of 0.6 mm in the direction perpendicular to the beam was observed, the position and dose distribution of the BP were generally correctly estimated.

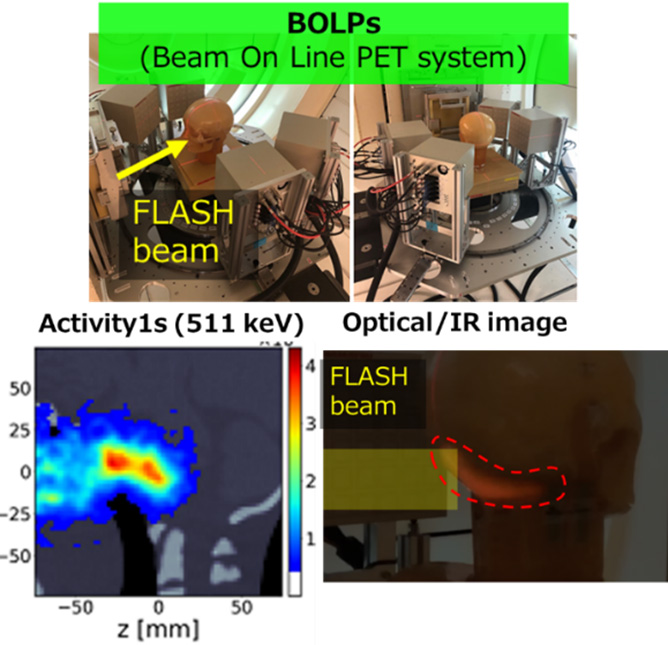
Regarding next-generation particle beam therapy, we are exploring (1) multi-ion particle beam therapy and flash therapy. Flash therapy is a new treatment method that minimizes damage (side effects) to normal cells by irradiating with a high-intensity particle beam for a short time. As a preclinical trial, we evaluated the efficacy and safety of multi-ion therapy using beams of He/C/O/Ne by measuring tumor growth delay and intestinal death in mice. We also progressed in constructing a flash irradiation system using heavy ion beams and a dose calibration system and conducted confirmation experiments of the flash effect from the perspective of intestinal death. In visualizing flash therapy with proton beams, we are challenging visualization of biological information, tumor areas, and surface dose distribution with visible light (long wavelength) imaging, irradiation areas and dose distribution with 511 keV gamma rays, and dose responsiveness visualization.

最終更新日:2024年7月1日
Last Update: 1 July 2024