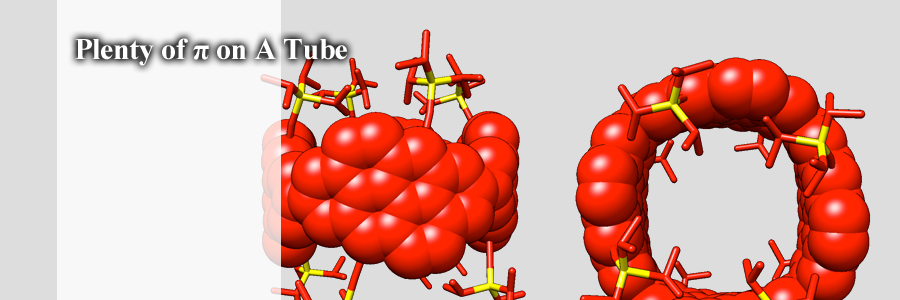
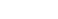Molecular nanotubes that possess periodic vacancy defects have been designed. The nanotube molecules, named phenine nanotubes (pNT), have been synthesized by connecting 40 benzene rings, which results in a cylindrical molecule with 240 π-electrons. In the crystalline solid state, the nanotube encapsulated multiple C70 fullerene molecules in interstitial and internal sites.
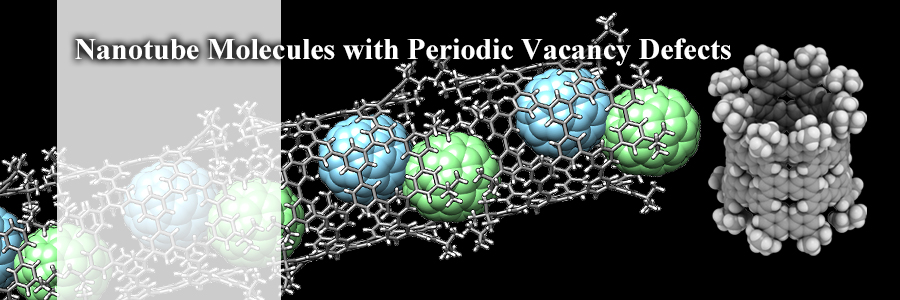
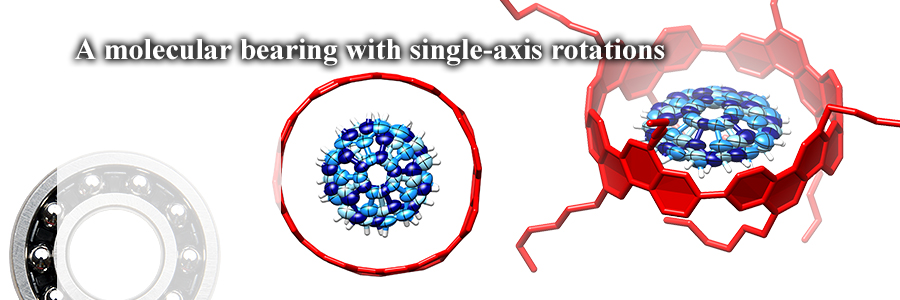
A bowl-in-tube molecular bearing was assembled solely by novel and weak hydrogen bonds, "CH-π hydrogen bonds". The hydrogen bonds were arrayed in concyclic manner to allow single-axis rotations of the entrapped bowl in the tube. Directionality of molecular motions has been controlled by a relay of weak yet directional forces.
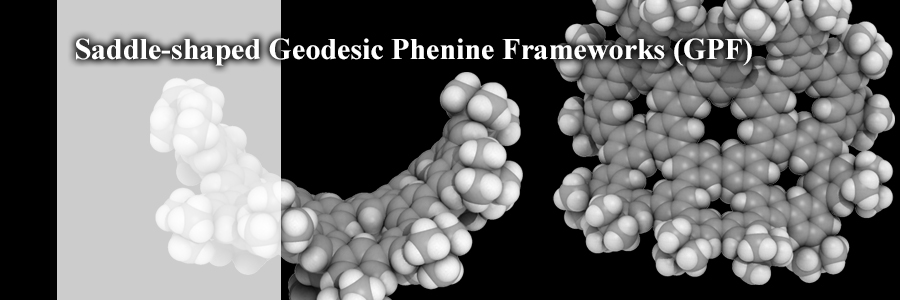
A saddle-shaped nanocarbon molecule was synthesized by adopting a geodesic phenine framework (GPF) design with one heptagon and seven hexagons of trisubstituted benzene (phenine). The unique saddle-shaped structure persisted, even though the local molecular structures fluctuated. The dynamic motions, persisted even in its stacked form, were reminiscent of swimming motions of a stingray's fin. GPF chemistry may deepen our understanding of curved graphitic sheets.
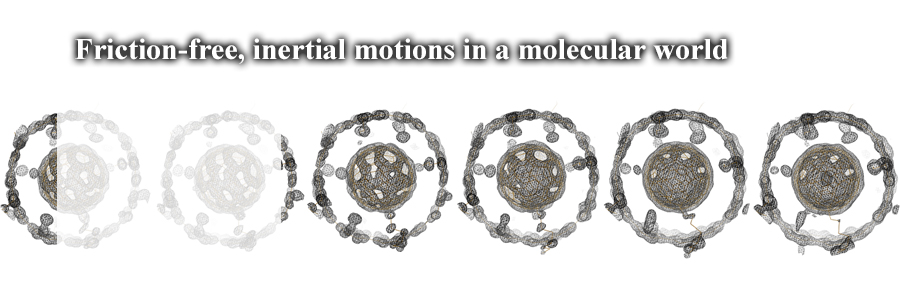
In the molecular world, typical, back-and-forth motions are known as Brownian motions. Here we discovered friction-free, inertial rotations of a spherical molecule bound tightly in a tubular molecule. The supramolecular system, named molecular bearing, was found inertially dynamic in the solid state, which is another unexpected discovery. Smooth, friction-free rotations in molecular bearings might lead us further new discoveries.
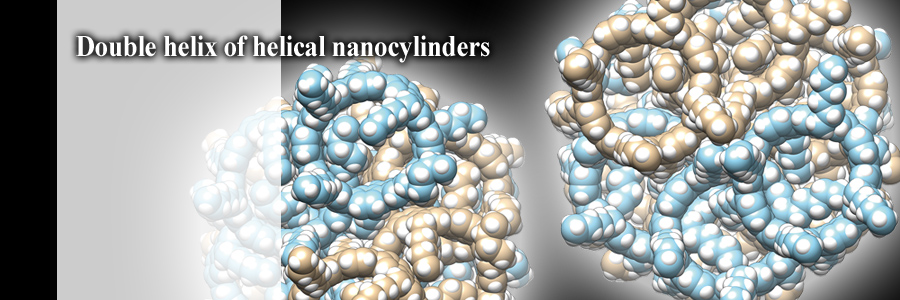
Rigid, nanosized cylindrical molecules formed a double helix in crystals. The right-handed nanocylinders formed a left-handed double helix, and the left-handed nanocylinders formed a right-handed double helix. When dispersed in solution, the nanocylinders recorded the largest dissymmetry factor ever recorded with organic molecules for circularly polarized luminescence.
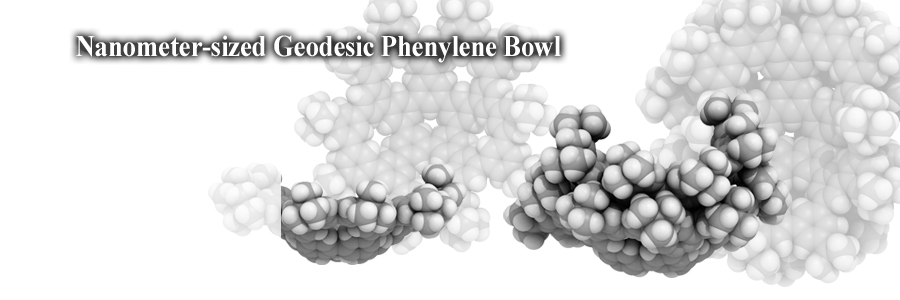
A nanometer-sized geodesic phenylene bowl was designed and synthesized by a combination of pentagon and hexagon arrays. The polygons mimic defective sites on nanocarbons to afford a molecule with a colander-like structure. The bowl formed a bowl-in-bowl assembly through a convex-concave stack. The results may shed a new light on curved π-systems.
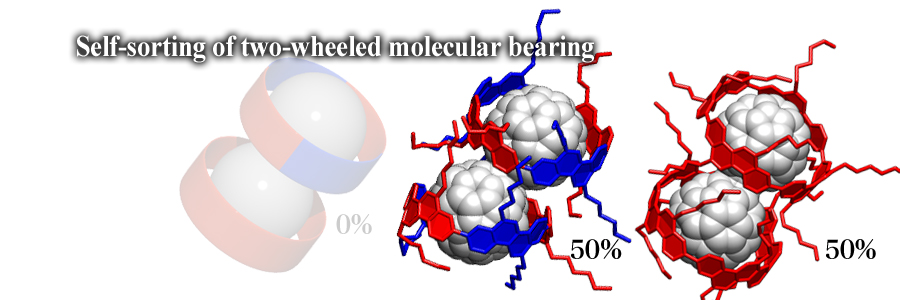
A two-wheeled molecular bearing comprising of two tubular receptors and one carbonaceous dumbbell-shaped guest was assembled. The tubular receptor recognizes the counterpart receptor, which allows for self-sorting of stereoisomeric receptors in a narcissistic manner. The discrimination of isomeric receptors is driven purely by van der Waals interactions, which may deepen our understanding of self-sorting behaviors taking place in Nature.
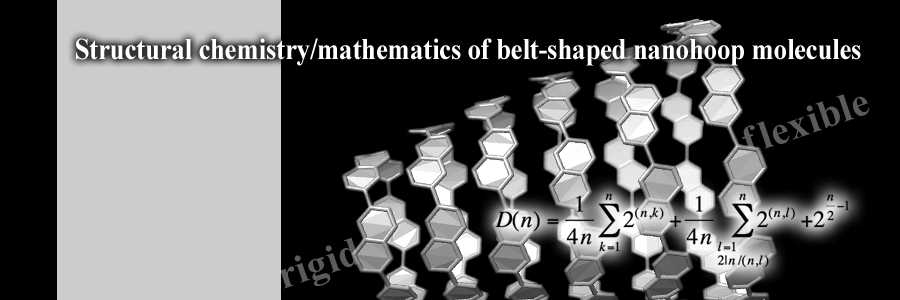
A belt-shaped cyclic array of aromatic panels, nanohoop, is expected to deepen our understanding of carbon nanotubes by providing discrete, segmental structures. The structural fluctuation/rigidity of nanohoops originate from the panel rotations, which have been revealed by a team of chemists and mathematicians. The fundamental knowledge of static and dynamic structures may facilitate the development of nanohoop chemistry.
A hydrocarbon macrocycle designed as a model molecule of defective graphene turned out to be an excellent material for negative electrodes of all-solid-state lithium rechargeable batteries. The capacity surpassed those of conventional graphite materials, which originated from nanoporous structures created by pore alignments of molecules.
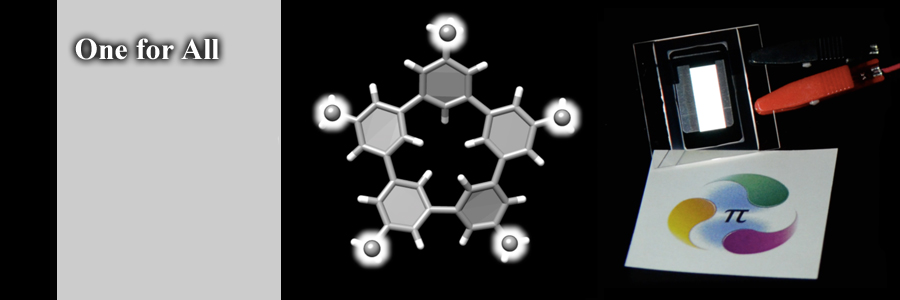
We developed one molecule for all the requisite functions in phosphorescent single-layer OLEDs. The simple molecule that is composed solely of hydrogen and carbon atoms transports hole, transports electron, provides a place for their recombination and fills the phosphor with excited energy to facilitate the emission in a quantitative manner.
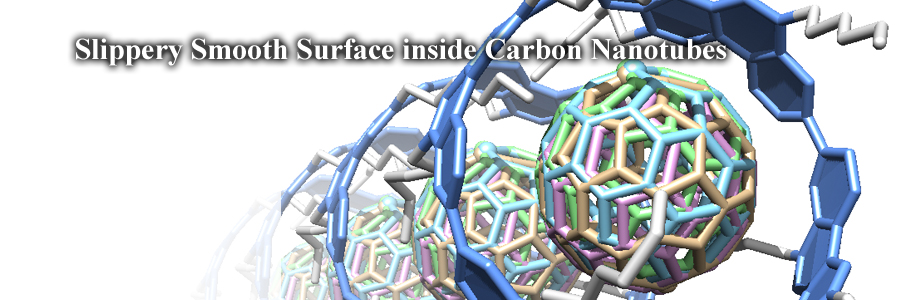
It was a slippery smooth surface of carbon nanotube molecules that allowed rapid dynamic motions of inner spherical guests. We now shed a (high-flux X-ray) light on the inner "mysterious world” of carbon nanotubes and revealed the atomic-level structures of a dynamic supramolecular system.
Geometric measures have been proposed to describe the length of finite carbon nanotube molecules. By utilizing a web-based applet for this “rulers”, anyone can readily compare the length of finite carbon nanotubes with discrete molecular structures.
A finite carbon nanotube molecule of a zigzag form has been chemically synthesized.
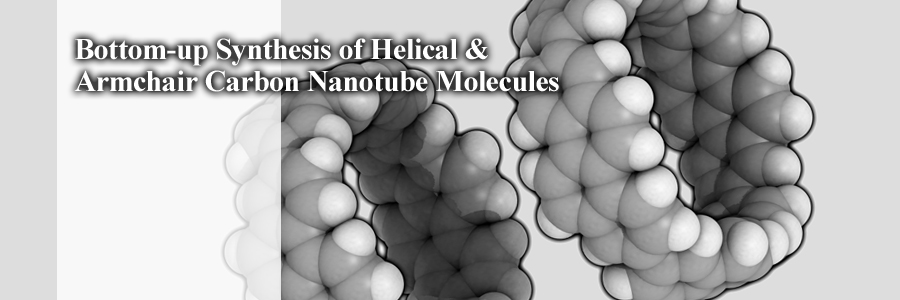
We now have all three nanotubes, that is, helical, armchair and zigzag, notably with an identical chemical composition.
They are mutually the true isomers of single-wall carbon nanotubes.
New tubular macrocycles with a maximum electrons of 120π have been synthesized from a abundantly available pigment.
We now have longer finite SWNT molecules of helical and armchair forms in quantity.
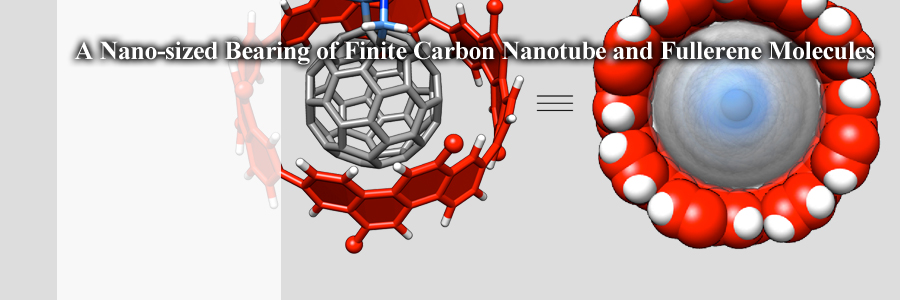
A carbonaceous bearing of a minimal form has been assembled with a finite carbon nanotube molecule and a functionalised fullerene molecule.
With the van der Waals attraction, the bearing holds the fullerene journal tightly to avoid its run-out motion, and the journal with a shaft rolls anisotropically in the bearing despite of the tight holding constraint.
Finite carbon nanotube molecules of helical and armchair forms have been synthesized.
The macrocyclic arylene molecules posses persistent belt-shape, which successfully mimics the wall of carbon nanotubes.