Research Results
High performance and economic efficiency
Hydrogen is activated at room temperature and under atmospheric pressure FY2016

- Seiji Ogo (Professor, International Institute for Carbon-Neutral Energy Research /Faculty of Engineering, Kyushu University)
- CREST
- Development of the Foundation for Nano-Interface Technology
"Energy Conversion via the interface with Hydrogen Activation Aqua Catalysis" Representative Researcher (2008-2013)
Consistently investigating'hydrogen'as an energy career
Recently, public and private sectors have paid attention to and discusse d'hydrogen 'as a representative for an energy career (energy-storage media) for the next generation. The world has now begun to recognize, with the background, in mind that fossil fuels are depleting and the catastrophic accident took place in Fukushima. A safe, clean, and sustainable energy supply is one of the significant challenges in the 21st century.
In light of this situation, the research group of Seiji Ogo has established the policy, and consistently focused on hydrogen, ascertaining the mechanism of hydrogen activation by an enzyme, and extracted electrons from hydrogen to develop energy utilization technologies. In 2007, the research group had already announced the first'model compound'that operated similar to the'hydrogen activating enzyme.'Later, they were selected as one of the representative researchers by CREST in 2008. They continued to work and finally succeeded in accomplishing the complete modeling of the hydrogen activating enzyme in February 2013.

Success of developing an artificial catalyst that does not use noble metals
(The Nikkan Kogyo Shimbun (News paper), February 8, 2013)
Launch for developing the artificial catalyst by modeling natural catalysts
The research by Professor Ogo et al. started with focusing on a protein that naturally acts as a catalyst*, in other words, a natura'l enzyme.' This hydrogen activating enzyme is called' nickel-iron hydrogenase.' The enzyme catalyzes 'proton (H+) reduction' and 'hydrogen oxidation,' under mild conditions (in room temperature and under atmospheric pressure). When this enzyme functions, hydrogen molecules are burned and utilized as energy. It was also known that the hydrogen molecules are utilized freely as hydride ion (H-: hydrogen with minus charge) or electrons.
With that in mind, the research group paid attention to this function in terms of its application to the research and development in hydrogen energy, and was successful in synthesizing an artificial catalyst using the function mentioned above in 2007: it was' nickel and ruthenium catalysts' which used the noble metal of ruthenium (Ru) as a catalyst instead of iron (Fe). This was the best functional model in terms of safety and quality performance among many artificial catalysts developed at that time. The following year, the research group was also successful in deriving hydrogen in room temperature and under atmospheric pressure using this catalyst.
* Substance that increases the speed of a chemical reaction but does not change itself or its action. It has specificity to create only the desired products.
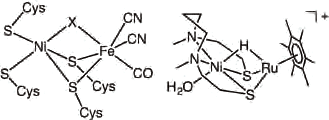
(Left) The structure of active center of Hydrogen Activating Enzyme"nickel-iron hydrogenase"
(Right) It was modeled structure of artificial Catalyst which synthesized"nickel and ruthenium catalysis"
The astonishing value of 'noble metal-free catalyst'
In February 2013, the research group of professor Ogo et.al. developed an artificial catalyst'nickel-iron catalyst'by modeling the'nickel-iron hydrogenase,' which functioned similar to the hydrogenase. Being different from the model that used the noble metal of ruthenium in 2007, the research group was successful in transferring electrons from hydrogen to electron acceptors (ferrocenium ion, methylviologen, etc.) by using a catalyst with iron in room temperature and under atmospheric pressure. After ascertaining the crystal architecture, the group also confirmed that the hydride ion (H-) that is generated after activating hydrogen bonds to iron, not to nickel. Previously, there was no one who was able to tell which one the hydride ion bonded to.
As a successful result the hydrogen activating using the catalyst with iron instead of previous model of ruthenium, this produced another benefit that was realized other than for just academic purposes. This is the cost. As of in February 2013, the market price of the noble metal of ruthenium used in the previous model was 240 yen/g, whereas that of iron was one- four thousandth of the price of ruthenium, 0.06 yen/g. It cannot be overstated that the ratio of metal price has a direct bearing on the complex catalyst price. This 'economic'value is significant since a large amount of catalysts is required for the use of fuel batteries, etc. In this sense it is a revolutionary development.
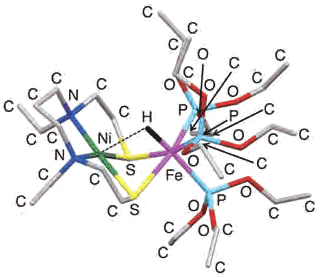
The crystal structure of'nickel-iron catalyst'
To what extent are the 'three themes' achieved?
The artificial modeling of the hydrogen activating enzyme, which started in 2007, has almost completely been accomplished through the success of this development of'nickel-iron catalyst.' Subsequently, it can be said that the research of hydrogen activating with the 'noble-metal-free catalyst,' as well as the breakthrough of the mechanism for hydrogen activating of natural enzyme, 'nickeliron hydrogenase', has made great progress.
As mentioned above, professor Ogo always emphasizes that following three themes have to be accomplished in order to solve energy problems in the 21st century: 1: Breakthrough function principle of life, 2: Developing the technique to abstract and apply the life function, 3: Overwhelming the life function. The first one, has been accomplished to some degree, since the breakthrough of the mechanism for hydrogen activating of 'nickel-iron hydrogenase' has continued. As for the second has been realized by the success of deriving electrons from hydrogen with the 'noble metal-free catalyst.' No.3 has to be worked on continually. In this regard, we may develop 'platinum-free fuel battery' with 'nickel-iron catalyst.' It is necessary to use 'platinum' for electrode catalysts in fuel batteries. However platinum is expensive, what is more, it is limited in supply. Alternative catalysts are awaited, such as 'nickel' or 'iron' that are rich in the amount of the deposit and the price is cheap.
