Research Results
A novel gene delivery system:
A key technology for advanced medicine FY2017

- Mahito Nakanishi (Director, Research Laboratory for Human Cell Engineering, Biotechnology Research Institute for Drug Discovery, National Institute of Advanced Industrial Science and Technology (AIST))
- START
- "The Technology of Creating Human Cell for Regenerative Medicine Using Stealth Type RNA Vector" Chief Researcher
A safe vehicle for gene delivery to advanced medicine
Gene therapy and regenerative medicine can become powerful strategies for treating incurable diseases. Gene delivery systems, which are technologies for delivering therapeutic genes and expressing them in human cells, are critical for realizing these advanced medicines.
The word "vector" means a vehicle for gene delivery. Recombinant animal viruses are the most popular vectors used in current medical applications. These viral vectors are generated by replacing pathogenic genes with therapeutic genes using recombinant DNA technology. On the other hand, classical viral vectors are not always suitable for advanced medical applications. Gene therapy often requires vectors that are capable of delivering large therapeutic genes and expressing them stably in tissue cells. However, most of the viral vectors can neither accept large genes nor express them stably. Stable gene expression generally requires integration of the therapeutic genes into the host chromosome.
However, this may cause undesired side effects, such as insertional mutagenesis and uncontrolled activation of oncogenes. Therefore, development of novel vectors satisfying these complex requirements has been desired.
In "Program for Creating STart-ups from Advanced Research and Technology" (START), JST supported a researcher who invented an innovative vector system called "Stealth RNA Vector (SRV)" and helped the researcher to start a venture company for commercializing this technology. The START program is unique among other grant systems because it supports the partnership of academic scientists and business promoters (venture capitalists), to facilitate research as well as business development. This approach should encourage realization of truly innovative products from risky but supreme technology, with the aid of public funds and know-how of business partners.
Stealth RNA Vector (SRV): an advanced gene delivery system for stable gene expression.
To achieve a stable gene expression, researchers have focused on precisely integrating exogenous genes into target sites of host genomic DNA. On the other hand, Dr. Mahito Nakanishi (National Institute of Advanced Industrial Science and Technology) chose a different approach: stabilizing RNA in the cytoplasm instead of stabilizing DNA in the nucleus. This approach has the advantage that RNA will not cause physical recombination with genomic DNA; therefore, we can avoid undesired side effects caused by the modification of genomic DNA. Furthermore, this RNA platform has the capacity to accept larger genetic inserts than other viral vectors.
A prototype of this vector was developed using a mutant strain of Sendai virus identified in Japan. Using this prototype vector (SeVdp vector), Nakanishi and colleagues, for the first time in the world, could achieve stable gene expression from an RNA platform. This technology has been evolved into SRV, which is capable of stable and simultaneous expression of up to ten genes on a synthetic RNA platform. An international patent application covering SRV technology was submitted in 2016.

SRV is an ideal system for generating induced pluripotent stem cells (iPS cells)
Human iPS cells, discovered by Dr. Yamanaka (a Nobel Prize winner in 2012), have the capacity to differentiate into various normal tissue cells, and they can be generated from peripheral cells of patient tissues. These characteristics make iPS cells an ideal resource for medical applications. However, methods for producing clinical-grade iPS cells have not yet been established, although researchers have been struggling hard to solve the problem. Originally, iPS cells were generated by introducing four reprogramming genes separately into the target cells, with the aid of retrovirus vectors. However, this procedure is unsuitable for clinical purposes because of safety concerns and low efficiency. On the other hand, the SeVdp vector and SRV generate high-quality iPS cells quite efficiently, as they can install all of the reprogramming genes on a single vector and express these genes simultaneously. In evaluation of various iPS technologies in EU StemBANCC, this technology was rated as the best by scientists involved in the evaluation.
Application of SRV technology is not limited to cell reprogramming; it is useful for the production of therapeutic antibodies and vaccines. Traditional production systems for the influenza vaccine need several months to scale up, whereas SRV technology can start large-scale production within a month after the genetic information for emerging influenza viruses is available.
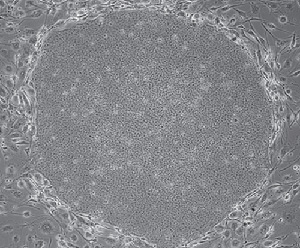
Microphotograph of an iPS cell
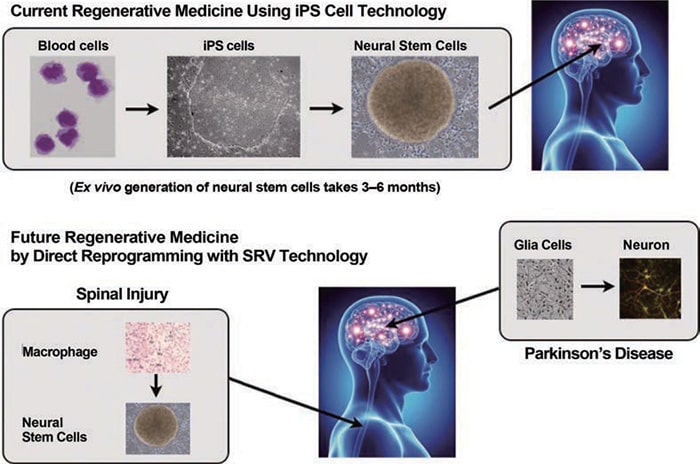
Application of Direct Cell Reprogramming in Regenerative Medicine
Future prospects: automatic production of clinical-grade iPS cells.
As described above, SRV technology should be a valuable tool in advanced medical applications and biopharmaceutical production. The START program supported the start-up of a venture company "TOKIWA-Bio, Inc." for realizing SRV technology throughout the world. TOKIWA-Bio transferred some of the rights of the patent on SRV from AIST and is now trying to establish the technology to allow full automatic production of clinical-grade iPS cells. In the near future, we may obtain high-quality iPS cells within a week, just by depositing a small quantity of blood sample into the machine. TOKIWA-Bio plans to make this technology the de facto standard for iPS-cell generation in the world.
Currently, it is difficult to produce iPS cells with high quality reproducibly because the cells are generated manually by a technical expert. SRV technology will make it possible to produce clinical-grade iPS cells automatically.
