Research Results
Temp ate for Carbon anotube anufacture
World First! Success in Synthesis of Carbon Nanobelts FY2018

- Kenichiro ltami (Professor, Nagoya University, Graduate School of Science / Director, Institute of Transformative Bio-Molecules)
- ERATO
- "Itami Molecular Nanocarbon Project" (2013 - 2019)
The Key to Carbon Nanotube Production "Carbon Nanobelts"
ln April 2017, a report released in an online bulletin of the scientific journal "Science" surprised researchers all over the world; within moments, the news had spread like wildfire globally. The research group led by Professor Kenichiro ltami had succeeded in synthesizing the first carbon nanobelt in the world.
Carbon nanotubes, atoms of carbon linked together in a tubular form, offer not only the characteristics of strength at low weight, but hold promise as a next-generation material, due to the fact that they conduct heat and electricity so well. However, its conductive property and strength depend on the diameter and structure of the tube. With conventional manufacturing methods, carbon nanotubes with different diameters and structures would mix and hinder the potential for industrial application. This led to carbon nanobelts garnering attention.
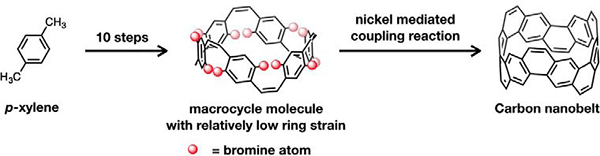
Process of carbon nanobelt synthesis
A dream substance pursued by researchers the world over
Because carbon nanobelts are subset of carbon nanotubes, if this work can be extended as a template, there is the potential to manufacture carbon nanotubes having structures corresponding to their design. The idea for carbon nanobelts first appeared in the literature around 60 years ago. Researchers from around the world had tried to synthesize them, but because of the significant strain* that arises from the benzene ring taking a tubular shape, synthesis was understood to be very hard. The research team of Professor Itami and others spent 12 tireless years to ultimately successd in symthesizing this dream molecule.
*Strain: a type of instability present when the bonds between atoms in a molecule form at unusual angles

Carbon nanotubes are made up only of carbon atoms
(the round parts of diagrams; the lines are chemical bonds)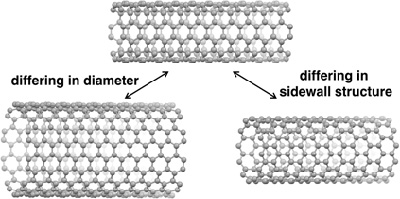
Carbon nanotubes with various diameters and structures. The various properties, such as electrical conductivity and strength, can differ.
The path opened up to success by evolutionary strategy
Professor ltami first researched carbon nanobelts in 2004, taking the opportunity afforded to him by invitation to the laboratory at Nagoya University of Professor Ryoji oyori, a recipient of the obel Prize for Chemistry. When interviewed, he thought that he had to give a research topic with a unique objective, so proposed the synthesis of carbon nanorings and carbon nanobelts, and he was hired. urthermore, he was selected by the JST "Sakigake" PRESTO program. His adviser supported him considerably, encouraging him to aim for a home run.
However, the road to carbon nanobelt synthesis is steep. He tried an enormous number of chemical reactions, but it did not look like he would ever arrive at his target destination due to a series of failures. The key for synthesis was a revolutionary strategy, wherein first one synthesizes ring molecules with no strain, which are then transformed into a tubular structure. Professor ltami's group succeeded in the synthesis via an 11 stage chemical reaction, starting with the cheap oil component para-xylene as the raw material, and using bromine and nickel at the final stage. The results of analyzing the synthesized carbon nanobelts revealed that in addition to having the same tubular structure as carbon nanotubes, they have a strong structure, throughout which electrons can pass.
Potential for carbon nanobelts to open up new fields
lf we can bond carbon using the carbon nanobelt as a template, and stretch it into a tubular form, it will bring about the next generation nanotubes. This could lead to major results in a variety of fields, such as the development of lightweight and bendable displays, and power-saving nanotubes, atoms of CP s, as well as high-performance batteries and photovoltaic cells.
Furthermore, carbon nanobelts themselves hold a variety of possibilities. A property of the ones recently created is that they glow red when struck by ultraviolet light; they have the potential to be used as a light-emitting or semiconductor material. Thanks to these revolutionary results, carbon nanobelts may open up a new field of "molecular nanocarbon science."

Left is structural diagram of carbon nanobelt. The red body in the center is the synthesized carbon nanobelt; when struck by ultraviolet light it glows red, as on the right.
