Research Results
Significant Progress in Implementation of Cell Transplantation Medicine to the Society
Development of a Cleanroom Unit for Humanoid Robots That Automate Cell CultureFY2025

- TAKAHASHI Koichi (Team Leader, RIKEN Center for Biosystems Dynamics Research)
- JST-Mirai Program
- Project Leader (Feasibility Study: 2018-2020, Full-scale: 2020-2024), Common Platform Technology, Facilities, and Equipment, mission area: "Accelerating life sciences by robotic biology"
- TAKAHASHI Masayo (Senior Advisor, Kobe City Eye Hospital)
- JST-Mirai Program
- Primary Co-researcher (Full-scale: 2020-2024), Common Platform Technology, Facilities, and Equipment, mission area: "Accelerating life sciences by robotic biology"
Development of the world's first robotic cell processing facility
The research group, which has been conducting research to automate processes in life science experiments, has developed and demonstrated a new approach to life science research that combines humanoid robots and artificial intelligence (AI). For example, the research group has succeeded in efficiently using induced pluripotent stem cells (iPS cells)*1 to produce retinal pigment epithelial cells (RPE cells)*2 without human intervention.
In the present research, in order to use the humanoid robot in actual clinical research on retinal regenerative medicine, the research group developed the world's first robotic cell processing facility*3 (R-CPF) that meets the cleanliness requirements of laboratory spaces required to prepare cells for transplantation (Fig. 1).
The use of humanoid robots at clinical sites may contribute to significantly advancing regenerative medicine.
*1 Induced pluripotent stem cells (iPS cells)
Pluripotent stem cells (undifferentiated stem cells that can differentiate into all tissue cells and germ cells that constitute the body) are artificially produced by reprogramming and conferring pluripotency to somatic cells such as adult skin cells through introduction of genes including Oct3, Sox2, and Klf4.
*2 Retinal pigment epithelial cells (RPE cells)
One of the cells that constitute the retina. Clinical research on regenerative medicine using induced pluripotent stem cell-derived retinal pigment epithelium are being conducted by RIKEN, Kobe City Eye Hospital, and others.
*3 Cell processing facility (CPF)
A dedicated cleanroom which maintains cleanliness at a level required for cell culture.
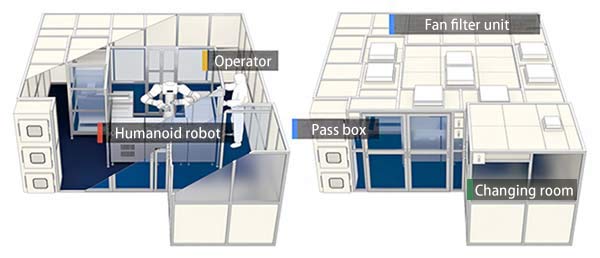
Fig.1 Overall View of the Robotic Cell Processing Facility
Challenges in materializing automation of cell manufacturing processes
In life science experiments such as those on cell culture, much labor and time are spent on simple tasks. Additionally, the speed and skill level of the work varies depending on the person performing the work, causing issues such as variability in productivity and reproducibility. To solve these issues, the research group has been conducting research on the automation of experiments using humanoid robots, with the aim of fully automating life science experiments using robots and AI.
Meanwhile, in order to actually use these robots in clinical regenerative medicine sites, the environment of cell manufacturing processes must be kept sterile. When humans manufacture and prepare cells manually, they do so in highly clean facilities called cell processing facilities (CPF), but there were no facilities that developed sterile environments for robots.
Development of a facility that enables automated cell preparation in a sterile environment
In this research, the research group designed a system that combines the general-purpose humanoid robot LabDroid Maholo*4, which is capable of high-precision motions in life science experiments, and the compact cleanroom unit All-in-one CP unit*5 to automate cell preparation at the cleanliness level required in clinical research.
First, the research group examined the layout of the R-CPF and employed a three-area structure, including a robot area where the robot directly works on the cells, and in the surrounding area, an area where operators operate the robot and a changing room that leads to the operator area (Fig. 2 left). By reference to the structures and facilities standards described in Approach to Sterile Operations in Cell Processing Facilities Based on the Act on Securing Safety of Regenerative Medicine of The Japanese Society for Regenerative Medicine (Fig. 2 center), the cleanliness level of each area was set at Grade A cleanliness level for the robot area, a sterile working area; Grade B cleanliness level for the operator area, a cleanliness control area; and Grade C cleanliness level for the changing room, a cleanliness control area. Ventilation facilities, fan filter units (FFU), were then installed to achieve these standards.
Subsequently, computers were used to verify if the planned facility provided an air-conditioned environment that meets the required cleanliness levels (Fig. 2 right). As a result, it was projected that each area would achieve the cleanliness level standards.
After such verification, the research group installed an R-CPF at Kobe City Eye Hospital. Actual cell culturing using the robot was performed, and achievement of the cleanliness levels in the respective areas were confirmed by monitoring airborne bacteria, falling bacteria, and surface-adhering bacteria at 33 locations inside and outside the R-CPF and examining the results (Fig. 3). At the same time, since the facility differs from previous CPFs, operators' routes and standard operating procedures (SOP), which affect cleanliness levels, were examined. As a result, all locations were found to meet the cleanliness levels, and SOPs including monitoring methods were established.
Furthermore, when iPS cell culture was performed for 10 days using the same cells and the same protocols, robotics motions equivalent to the same motions of human operators, and the same cell quality control tests as those in previous clinical research, cleanliness levels at all locations were determined to be within the standard values. These results demonstrated that R-CPF can be used for cell preparation in clinical research.
*4 General-purpose humanoid robot LabDroid Maholo
Humanoid robot system for life science experiments developed by Robotic Biology Institute Inc. (subsidiary of YASKAWA Electric Corporation). The same experiment equipment used by humans in experiments are placed around YASKAWA Electric Corporation's industrial 7-axis dual-arm robot. Maneuvers in experiments that humans perform by hand, such as pipetting and opening and closing of incubator doors, can now be performed by robots.
*5 All-in-one CP unit
A cleanroom unit developed by DAI-DAN CO., LTD. Functions required of CPFs, such as changing rooms and cell preparation rooms, are unitized, allowing space-efficient and easy installation.
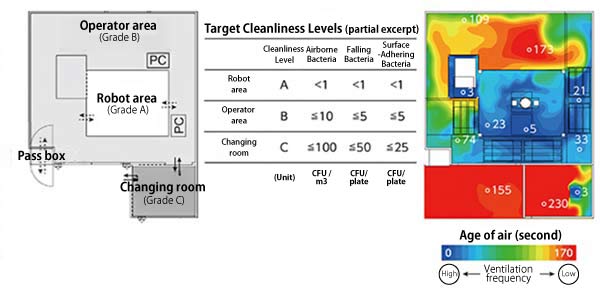
Fig.2
Layout and Air Conditioning Simulation of the Robotic Cell Processing Facility
(Left) Floor plan of the facility. The facility comprises a robot area, operator area, and changing room. Solid arrows indicate the movement lines of people, and dotted arrows indicate the movement lines of cells, reagents, consumables, and other materials. The robot is installed with the front side facing down. The cleanliness level of the facility was set at Grade A for the robot area, Grade B for the operator area, and Grade C for the changing room.
(Center) Target values of cleanliness levels. Excerpt from Approach to Sterile Operations in Cell Processing Facilities Based on the Act on Securing Safety of Regenerative Medicine of The Japanese Society for Regenerative Medicine. The number of bacteria was measured according to the number that could form colonies on the medium (colony forming unit [CFU]).
(Right) Computational fluid dynamics (CFD) simulation results on airflow. Age of air (in second units) at a location that is 1 m in height from the floor. The robot area including the area behind the robot, a sterile working area, showed a low age of air (represented by cool colors in the heat map), indicating that this space is frequently ventilated.
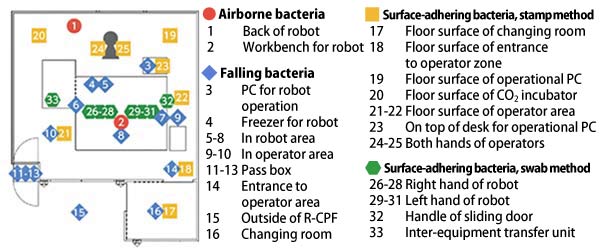
Fig.3 Monitoring Locations for Airborne Bacteria, Falling Bacteria, and Surface-Adhering Bacteria Monitoring of airborne bacteria, falling bacteria, and surface-adhering bacteria was performed at 33 locations inside and outside the R-CPF. Two methods including the stamp method (a method in which the surface is brought into contact with the agar medium) and the swab method (a method in which the surface is wiped) were used to detect surface-adhering bacteria.
Aiming for a high-speed, highly-sensitive, as well as low-cost automatic detection device
The research results enabled the implementation of robots for cell culture processes in clinical research, which previously could not be realized due to the lack of a suitable work environment. This will lead to reducing the burden of simple tasks in research processes that were previously performed by the human hand, reducing the cost of operator training including that for manual procedures, and improving the reproducibility of operations. As a result, the cost of regenerative medicine and cell therapy will likely decrease.
Further, when applying the results of basic research to clinical research, the people in charge of the respective processes in the research results often differ, disrupting the handover of technical skills from basic research to clinical research in some cases. The robot used for the R-CPF in this research employs the same humanoid robot used in basic research, and therefore, the culture conditions that the robot discovered and established in basic research can be implemented as is at the clinical site. Consequently, the person-to-person transference of technical skills is no longer necessary, rendering the handover from basic research to clinical research simple. Going forward, this will likely advance AI and robotics-based automation of life science experiments and further accelerate the development of regenerative medicine treatments.
- Life Science
- Research Results
- Japanese
