Research Results
Expected potential contribution to the development of future anti-aging and cancer prevention methods
Elucidated the mechanism of induction of senescent cell death in the longest-lived rodents, naked mole-ratsFY2024

- MIURA Kyoko (Professor, Faculty of Life Sciences, Kumamoto University)
- Fusion Oriented Research for Disruptive Science and Technology (FOREST)
- "Elucidation and application of homeostasis mechanisms unique to long-lived rodents" (2022- up to 10 years)
Discovered senescent cell death unique to naked mole-rats
A research group led by Professor Kyoko Miura and Assistant Professor Yoshimi Kawamura at the Faculty of Life Sciences, Kumamoto University, discovered that induction of cellular senescence in fibroblasts*1 of naked mole-rats (NMRs), the longest-lived rodents with resistance to aging and carcinogenesis (Fig. 1), leads to cell death that is not observed in other species. Furthermore, they found that serotonin metabolic regulation and vulnerability to hydrogen peroxide (H2O2)*2, which are unique to NMRs, work as the mechanism for senescent cell death, and that this mechanism also occurs in vivo.
Previously, some studies have shown that fibroblasts from NMRs can undergo cellular senescence under several conditions. However, in the tissues of aged NMRs, the expression of senescence-associated genes is low, suggesting the possibility of some mechanism unique to NMRs that suppresses the accumulation of senescent cells in their body. The results of this research suggest that, in NMRs, senescent cells undergo cell death through species-specific regulation of serotonin metabolism and vulnerability to H2O2, preventing the accumulation of senescent cells in vivo, which may contribute to the resistance to aging and carcinogenesis in this species.
Further research is expected to lead to the development of safer drugs to remove senescent cells.
*1 Fibroblasts
A type of cells that make up connective tissue and produce extracellular matrix such as collagen, contributing to the maintenance of the structure of skin tissue and organs. Fibroblasts also play an important role in wound healing and tissue repair.
*2 Hydrogen peroxide (H2O2)
A type of reactive oxygen species generated during energy metabolism. It oxidizes and damages DNA, fatty acids, and biological membranes, etc.

Fig.1 Naked mole-rat
Rodents that inhabit Africa. They have longevity and resistance to carcinogenesis and aging.
The longest-lived rodents, naked mole-rats, with resistance to aging and carcinogenesis
Generally, senescent cells have irreversibly stopped proliferating, are resistance to cell death, and tend to accumulate in the body with aging. It has been reported that accumulated senescent cells secrete various factors that induce inflammation, thereby promoting chronic tissue inflammation, aging, and various age-related diseases such as cancer.
NMRs are the longest-lived rodents living underground in the African savannas and are known for their exceptional resistance to aging and carcinogenesis. Their maximum lifespan is no less than 37 years, approximately 10 times longer than that of mice of similar size.
Although the resistance to aging and carcinogenesis in NMRs has been studied mainly in vitro, focusing on stress tolerance, DNA repair mechanisms, protein stability, and translation accuracy, the mechanisms of resistance to aging in NMRs in vivo have remained largely unexplored.
The Department of Aging and Longevity Research, Faculty of Life Sciences, Kumamoto University, the only laboratory in Japan that breeds NMRs, has been engaged in research on the resistance to aging and carcinogenesis in NMRs.
Serotonin accumulating in fibroblasts of naked mole-rats is the key to senescent cell death
To investigate the fate of senescent cells in NMRs, the research group led by Professor Miura et al. compared and analyzed the process with that in mice, which have a short life span of 2 to 3 years and undergo cellular and individual senescence. Cellular senescence was induced by adding low-concentration doxorubicin (DXR)*3 to fibroblasts from mice and NMRs. The results showed that both mice and NMRs exhibit characteristics of cellular senescence, but cell death, including apoptosis*4, increases gradually only in NMR cells (Fig. 2). This cell death also occurred when the gene INK4a*5, which plays an important role in cellular senescence, was artificially expressed. It was also demonstrated that this cell death is not mediated by the p53 gene*6, which is important for inducing acute apoptosis, but rather by the activation of the RB gene*7, which is crucial for cellular senescence induction by being dephosphorylated downstream of INK4a.
*3 Doxorubicin (DXR)
A type of anticancer drug. It enters DNA base pairs and inhibits DNA synthesis, replication, and transcription.
*4 Apoptosis
One of the mechanisms of programmed cell death. Apoptotic cells undergo nuclear and cytoplasmic fragmentation, but their ability to induce inflammation is limited because the cell membrane is preserved and intracellular components do not leak out.
*5 INK4a
A cyclin-dependent kinase inhibitor. It inhibits cyclin-dependent kinases, which are important in the cell cycle, and dephosphorylates RB, described below, resulting in the arrest of the cell cycle.
*6 p53 gene
One of the cancer suppressor genes that is activated when cells are exposed to stress or DNA damage. Its functions include inducing DNA repair, arresting cell growth, and apoptosis.
*7 RB gene
A cancer suppressor gene. It is phosphorylated and inactivated by cyclin-dependent kinase during the cell cycle progression, but when cyclin-dependent kinase is inhibited by INK4a, it is dephosphorylated and binds to and inhibits E2F, which is important for cell cycle progression, resulting in cell cycle arrest.

Fig.2 Cellular senescence induction using doxorubicin (DXR)
(Left) Pictures of cellular senescence induced by adding low-concentration doxorubicin (DXR) to fibroblasts from mice and NMRs. The yellow arrows indicate dead cells.
(Right) As days passed, cell death, including apoptosis, increased significantly only in the cells from NMRs. ns: No significant difference. *: Significant difference.
Examination of metabolites during the cellular senescence induction revealed that the NMR fibroblasts had an accumulation of serotonin prior to senescence induction, which was not observed in mouse fibroblasts. After cellular senescence induction, serotonin decreased while its metabolite, 5-hydroxyindoleacetic acid (5-HIAA), increased (Fig. 3, left).
Serotonin is known to produce large amounts of hydrogen peroxide (H2O2) when it is metabolized to 5-HIAA by monoamine oxidase (MAO) (Fig. 3, right). In fact, unlike mouse cells, senescent NMR cells showed increased protein levels of MAO.
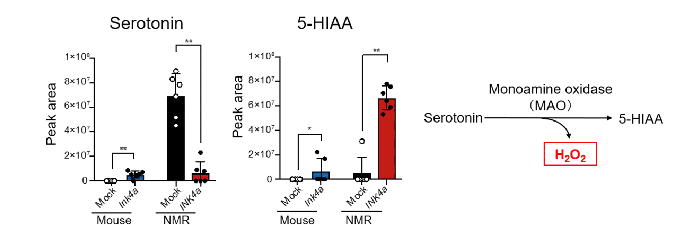
Fig.3 Changes in serotonin and its metabolite 5-HIAA levels before and after cellular senescence induction
(Left) Analysis of serotonin and 5-HIAA levels in mouse and NMR fibroblasts in which cellular senescence was induced by forced expression of INK4a. NMR cells showed higher serotonin levels before cellular senescence induction, and higher 5-HIAA levels after cellular senescence induction.
(Right) Serotonin is metabolized by MAO to 5-HIAA. In this process, a large amount of H2O2 is produced.
NMRs have a significantly lower level of activity of an antioxidant enzyme (glutathione peroxidase) that reduces H2O2 to water, and it has been known that NMR fibroblasts are difficult to maintain under normal oxygen conditions and show a marked vulnerability to H2O2 compared to mouse fibroblasts. This suggested that H2O2 produced during senescence induction may cause cell death in NMRs.
Therefore, the research group induced cellular senescence, and then added antioxidants that inhibit reactive oxygen species, including H2O2, or MAO inhibitors. As a result, cell death in NMRs was significantly suppressed (Fig. 4).
These results demonstrated that MAO plays an important role in cell death in NMR fibroblasts after senescence induction.
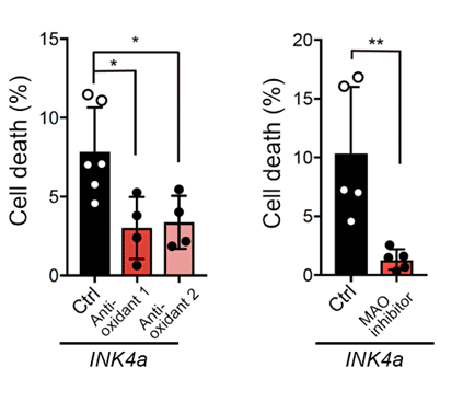
Fig.4 Cell death was inhibited by adding antioxidants and monoamine oxidase inhibitors
Treatment of INK4a-transfected NMR fibroblasts with antioxidants or MAO inhibitors significantly inhibited cell death.
Furthermore, to determine whether a similar mechanism exists in vivo in NMRs, the research group administered bleomycin,*8 a DNA-damaging agent, into the lungs of mice and NMRs to induce cellular senescence. The results showed that acute cell death occurred in both mice and NMRs. However, on day 21, senescent cells accumulated significantly in mice, whereas there was a significant increase in cell death in NMRs.
In addition, administration of MAO inhibitors to NMRs inhibited cell death and increased cellular senescence (Fig. 5). These findings suggest that the cell death resulting from serotonin metabolism by MAO is involved in preventing the accumulation of senescent cells in the lungs of NMRs.
*8 Bleomycin
An anticancer drug. It induces DNA breaks.
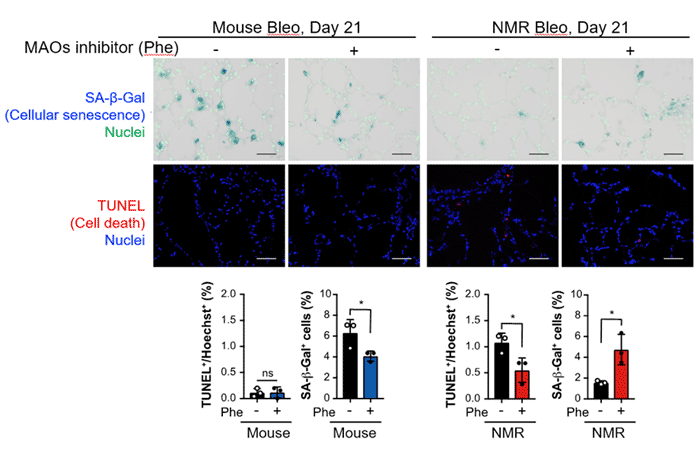
Fig.5 In the lungs of naked mole-rats after cellular senescence induction, administration of monoamine oxidase inhibitors suppressed cell death and increased cellular senescence
After cellular senescence induction by administering bleomycin to the lungs of mice and NMRs, cell death increased only in NMRs on Day 21. The addition of MAO inhibitors suppressed cell death and increased senescent cells in NMRs.
Expected contribution to the development of safer "senolytic drugs"
This study demonstrated that induction of cellular senescence in NMR fibroblasts causes cell death that is not observed in other species, and that this mechanism involves species-specific regulation of serotonin metabolism and vulnerability to hydrogen peroxide (H2O2). This process was also observed in vivo in NMRs, resulting in a contribution to the inhibition of senescent cell accumulation. This mechanism of preventing senescent cell accumulation may contribute to the resistance to aging and carcinogenesis in NMRs.
In recent years, development of "senolytic drugs" that remove senescent cells has been in progress. It has been reported that senescent cells are involved in tissue repair and other homeostatic functions of the living body; therefore, it is necessary to conduct research from the perspective of the safe removal of senescent cells for safer new drug development. On the other hand, NMRs are assumed to have acquired an innate and highly secure senolytic mechanism in the process of evolution by coordinating and utilizing their vulnerability to H2O2 and the "serotonin metabolic switch" that is activated during cellular senescence.
Future studies on the senolytic mechanism in NMRs may help to identify which senescent cells are suitable for elimination, potentially contributing to the development of safer "senolytic drugs".
- Life Science
- Research Results
- Japanese
