Research Results
World’s first demonstration case that overturns the conventional view of evolution
Successful laboratory evolution of E. coli into an insect’s bacterial symbiont in a short period of timeFY2024

- FUKATSU Takema (Prime Senior Researcher, Bioproduction Research Institute, National Institute of Advanced Industrial Science and Technology)
- ERATO
- Research Director (2019-2024) “FUKATSU Evolving Symbiosis Project”
Successful laboratory evolution of E. coli into an insect symbiont
ERATO “FUKATSU Evolving Symbiosis Project” has succeeded in evolving E. coli into bacterial symbionts that are essential for insects using its originally established system of symbiotic evolution of insects and E. coli (the artificial insect-E. coli symbiotic evolution system). Specifically, bacterial symbionts were removed from Plautia stali Scott (referred to below as stinkbugs), which cannot survive without bacterial symbionts, and they were infected with rapidly evolving E. coli and continuously reared and maintained, and as a result, it was found that, in a short period of between several months to about 1 year, E. coli can evolve into bacterial symbionts that are essential for supporting the survival of stinkbugs, due to a single mutation*1 in E. coli.
*1 Mutation
A change that occurs to the carrier of genetic information in an organism (often genetic DNA). This includes various changes, such as base substitution, insertion, deletion, inversion or translocation.
Pursuing the establishment of an advanced symbiotic relationship between the host and microorganism
Symbiotic relationships between microorganisms and flora and fauna, including humans, are deeply related to the acquisition, maintenance and evolution of biological functions, and have such a pervasively important role that it is no exaggeration to say that living organisms cannot survive without symbiotic relationships. In recent years, research relating to symbiosis has made large advances internationally, but conventionally, research has focused almost completely on existing symbiotic relationships, and there is an extremely limited amount of research taking the approach of actual empirical observation of symbiotic evolution, which is evolution leading to the acquisition of a symbiotic relationship, to answer the question of how a symbiotic relationship starts and is established. In particular, the evolutionary origin of a sophisticated symbiotic relationship with a microorganism that is essential for the survival of the host organism is an important research topic that remains untouched.
In recent years, the field of experimental evolution, which aims to elucidate the processes and mechanisms of evolution in real time in the laboratory, mainly in microorganisms, is becoming increasingly active. However, the evolution of advanced mutualistic*2 microorganisms that are essential for the survival of the host organism generally occurs over a long period of years, and demonstrations artificially reproducing this evolution in the laboratory were thought to be infeasible.
*2 Mutualistic
Symbiotic in a way that mutually benefits each organism in the symbiotic relationship.
Artificially inducing symbiotic evolution using stinkbugs and E. coli
This project aimed to demonstrate the processes and mechanisms of the evolution of advanced mutualistic microorganisms by means of an experimental evolution system using stinkbugs and E. coli. The research group of Takema Fukatsu, Prime Senior Researcher, Bioproduction Research Institute, National Institute of Advanced Industrial Science and Technology, has a track record of leading international research into a variety of insects, including stinkbugs, which are known as important agricultural pests, and their symbiotic microorganisms.
In order to survive, stinkbugs require bacterial symbionts stored in the symbiotic organs*3 that develop in the posterior intestinal tract, and in an uninfected state without bacterial symbionts, the growth of larvae is markedly delayed, and almost all die off without becoming adults. In infection replacement experiments assessing the influence of exchanging bacterial symbionts between stinkbug populations distributed across the regions of Japan, when bacterial symbionts were removed from stinkbugs and the stinkbugs were instead made to ingest E. coli, their growth was markedly delayed, but a very small number (around 5% to 10%) of small adults with abnormal body color exhibited emergence. E. coli is usually a bacterium that inhabits the intestines of mammals including humans, and should not have any symbiotic relationship with stinkbugs, but it was found to have the minimal ability necessary to symbiotically support the survival of stinkbugs (Fig. 1).
*3 Symbiotic organs
Specialized organs of the host organism for storing symbiotic microorganisms. In the case of stinkbugs, there is a sequence of many bag-shaped structures called ceca in the posterior digestive tract that store symbiotic microorganisms inside them.

Fig.1. Stinkbugs infected with bacterial symbionts (above) and stinkbugs infected with E. coli (below)
(A, B) Emerged adults infected with each bacterium. Large differences in body color and body size can be seen.
(C-F) The posterior intestinal tract is a symbiotic organ, and bacterial symbionts are stored inside the ceca, which appear yellow. When stinkbugs were made to ingest E. coli, the growth of the symbiotic organ is poor and they become colorless, but E. coli is observed in the same locations.
(G, H) The mother spreads bacterial symbionts on the surface of the eggs, and the hatched larvae acquire the bacterial symbionts by sucking the surface of the eggs. Mothers infected with E. coli also spread E. coli on the surface of the eggs they laid.
The aim of this project was to infect stinkbugs with E. coli, which is continuously subcultured, to evolve the original qualities of the bacterial symbionts in a laboratory. However, symbiotic evolution cannot be expected to occur simply by infecting stinkbugs with E. coli. Accordingly, the project used fast-evolving E. coli*5, whose rate of molecular evolution*4 was accelerated to around 100 times the usual rate by destroying DNA repair enzymes to increase the spontaneous mutation rate of genes. Non-infected stinkbug larvae were made to ingest this fast-evolving E. coli, and multiple experimental evolution systems with selections for growth speed and for emerged adult body color were prepared, and the host stinkbugs were continuously infected, reared and maintained for approximately 2 years, which corresponds to at least 10 generations of the host stinkbugs (Fig. 2).
*4 Rate of molecular evolution
The speed at which genes evolve. The rate of molecular evolution increases proportionally with the spontaneous mutation rate.
*5 Fast-evolving E. coli
E. coli whose rate of molecular evolution has been accelerated by genetic manipulation to increase the rate of accumulation of spontaneous mutation. This research used an E. coli strain from which the DNA mismatch repair enzyme gene mutS had been deleted.
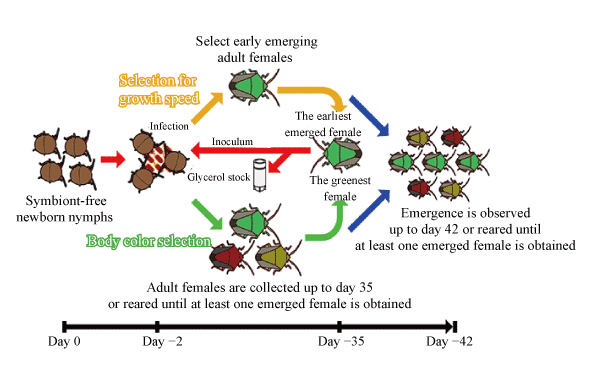
Fig.2. Design of the symbiotic evolution experiment
In selection based on growth speed, the adults that emerged most quickly were chosen, and in selection based on body color, the adults with the greenest body color were chosen, and the symbiotic organs of these adults were resected and the next generation of non-infected larvae were made to inject the E. coli inside.
The result was that an evolution lineage with markedly increased (30% to 80%) rate of emergence appeared in approximately 1 year (generation 7) in selection based on adult body color and in only 2 months (generation 2) in selection based on growth rate. As the emergence rate of these lineages increased, the traits of the stinkbugs tended to approach those of normal stinkbugs storing the original bacterial symbionts, with their body color becoming greener and their body size increasing (Fig. 3).
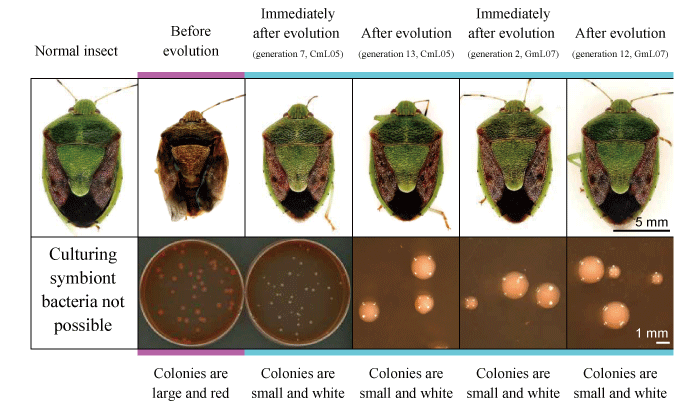
Fig.3. Appearance of stinkbugs infected by fast-evolving E. coli and changes in the shape of E. coli colonies
In addition, various changes were observed not only in the host stinkbugs but also in the E. coli. In evolving E. coli strains that improved the survival and body color of the host in the same way as the original bacterial symbiont, marked characteristics such as reduced production of extracellular matrix, reduced proliferation rate, smaller cell sizes and loss of motility were observed.
Accordingly, the whole genome sequences were determined and compared using a next-generation sequencer*6 and the spontaneous mutations that were expressed in the genomes of E. coli as symbiotic evolution progressed were comprehensively evaluated. From the results, in the evolved E. coli strain selected based on body color and in the strain based on selection of growth speed, the function-loss spontaneous mutation of genes of the broad transcription regulatory system*8 involved in carbon catabolite repression*7 was identified as a candidate. To confirm that these gene mutations are the cause of symbiotic evolution, the spontaneous mutations of these genes were introduced into wild-type E. coli, and changes similar to those in the E. coli that underwent symbiotic evolution in stinkbugs, such as reduced production of extracellular matrix, reduced proliferation rate, smaller cell sizes and loss of motility were observed (Fig. 4). In addition, when non-infected stinkbug larvae were made to ingest these E. coli strains, increased emergence rates and greener body colors were reproduced. In other words, the results showed that, due to the occurrence of a spontaneous mutation that removes the function of carbon catabolite repression so that no switching of metabolism occurs even if glucose is depleted, E. coli changes so that it improves the survival and body color of stinkbugs.
These results overturn conventional expectations and make it clear that evolution of mutualistic bacteria that are essential for the survival of the host organism can occur rapidly and simply, and that E. coli can become a bacterial symbiont that is essential for supporting the survival of stinkbugs due to just one spontaneous mutation in its genome.
*6 Next-generation sequencer
Equipment that determines the DNA base sequence and can obtain a vast amount of base sequence information, amounting to tens of millions or billions of base pairs, in a single analysis.
*7 Carbon catabolite repression
A broad transcription regulatory system found in species of bacteria such as E. coli, with a mechanism that suppresses gene expression of the system decomposing carbon sources that are difficult to metabolize when easily metabolized carbon sources such as glucose are available until these easily metabolized carbon sources are consumed.
*8 Broad transcription regulatory system
When a single modulating gene controls the expression or function of multiple gene clusters, the modulating gene is called a broad transcription factor and the whole system is called a broad transcription regulatory system.
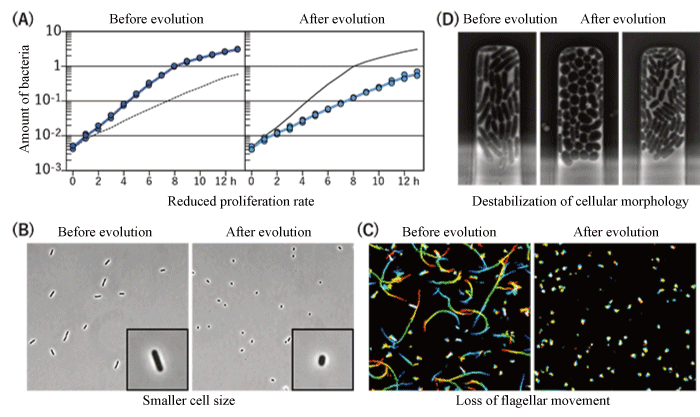
Fig.4. Changes in fast-evolving E. coli before and after symbiotic evolution
Uncovering the mechanism of symbiosis that is common to a broad range of organisms
The use of the artificial insect-E. coli symbiotic evolution system established in this project is expected to lead to rapid progress in understanding the processes and mechanisms of symbiotic evolution, which have long remained unexplained. This project has also investigated the mutual symbiotic evolution system involving non-infected mice as hosts, in addition to insects. Approaches such as this are expected to contribute to future healthcare or maintenance of health through control of intestinal bacteria, by uncovering the differences and similarities of the intestinal symbiosis mechanisms of both invertebrates and vertebrates.
- Life Science
- Research Results
- Japanese
