Research Results
Solving food and environmental problems
New recycling system converts plastic into fertilizerFY2023

- AOKI Daisuke (Associate Professor, Department of Applied Chemistry and Biotechnology, Chiba University)
- PRESTO
- Researcher (2018-2022), "Fusion of different polymer topology utilizing topology transformation reaction" in the research area of "Topological Materials Science for Creation of Innovative Functions"
Transforming plastic into fertilizer to grow plants
In this study, plant-based plastic was degraded with ammonia solution and successfully converted into fertilizer that promotes plant growth. Experiments proved that urea*1 and sugar-derived compounds generated by ammonia-induced degradation of sugar-derived plastic (polycarbonate) composed of carbonate bonds*2, can actually promote plant growth, demonstrating a "recycling system that transforms plastic into fertilizer" (Fig. 1).
*1 Urea
Urea has long been used in agriculture as a chemical fertilizer to grow plant leaves and stems. It is the first organic compound synthesized from inorganic compounds.
*2 Sugar-derived plastic (polycarbonate) composed of carbonate bonds
A general term for polymers that are obtained when monomers (the smallest units that make up plastics) are linked together continuously via carbonate bonds. Polycarbonate, which is obtained from petroleum-derived bisphenol A as a monomer, is widely used as an engineering plastic with excellent heat resistance and transparency.
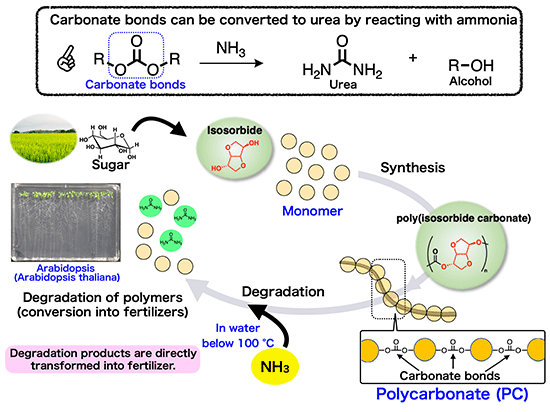
Fig. 1 Conceptual diagram of a recycling system that produces fertilizer from plastic
Plastic recycling has stagnated over the years
Plastic (a polymer material) is indispensable to our daily lives. The process of reusing used plastic by returning it to the starting material (the starting raw material for synthesis) is called chemical recycling*3, and has been studied for a long time. However, the efficiency of recycling waste plastic has not yet increased dramatically. More than 70% of plastic still goes to waste, with less than 15% recycled. In order to create a recycling-oriented society that is also compatible with the Sustainable Development Goals (SDGs), there is a need for a new recycling system that adds value to the conventional recycling process, as well as improvements in the cost and efficiency of plastic processing.
*3 Chemical recycling
Recycling of resources after compositional conversion by chemical reaction. In the case of plastic (a polymer material), the material is returned to a monomer or oligomer linked with a few monomers, and then polymerized again to regenerate the original polymer material or a new polymer.
Confirmation that recycling plastic produces chemical fertilizers
Ammonia (NH3), which came to be synthesized by the ammonia synthesis method (Harbor-Bosch method) *4 invented in the early 20th century, was converted into a chemical fertilizer (urea) for growing wheat and other crops, and dramatically increased food production.
Associate Professor Aoki and his colleagues focused on the fact that plastic with carbonate bonds (polycarbonate) reacts with ammonia, and is converted into chemical fertilizer (urea). The aim of this study was to establish a new recycling system in which urea, produced from degradation of plastic with ammonia, would be used as a fertilizer.
The ideal plastic for demonstrating the newly developed recycling system was polycarbonate, which is synthesized using biomass resource isosorbide*5 as a monomer (the smallest unit of plastic). Polycarbonate was expected to be an engineering plastic material with high heat resistance, excellent mechanical strength and transparency, and had the advantage of being in great demand for recycling.
In this study, isosorbide (a biomass resource) was used as a starting point for a plastic recycling system, and polycarbonate was synthesized. The polycarbonate was then degraded with ammonia, which resulted in a completely homogenous solution after 24 hours (Fig. 2). In addition, the amount of urea formed and the degraded products were evaluated from various aspects, and it was revealed that the polycarbonate can be completely degraded into urea and isosorbide. As a result, after optimizing conditions such as ammonia concentration and reaction temperature, the polycarbonate was completely degraded into urea and isosorbide within 6 hours (Fig. 3).
*4 Ammonia synthesis method (Harbor-Bosch method)
A process (developed by Fritz Haber and Carl Bosch in 1906) that uses iron-based catalysts to produce ammonia by directly reacting nitrogen from the air with hydrogen. It is said that this technology enabled the mass production of chemical fertilizer, which led to a rapid increase in food production and made it possible to cope with population increase.
*5 Biomass resource isosorbide
One of the renewable (always present in nature) biogenic monomers. It is obtained by chemical conversion of glucose.
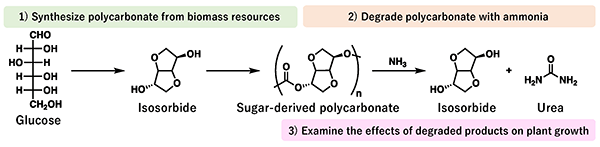
Fig. 2 Synthesis of polycarbonate from isosorbide
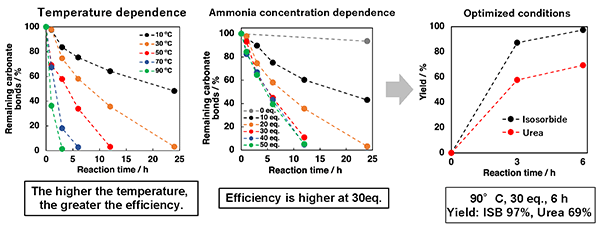
Fig. 3 Amount of residual carbonate bonds and amount of urea formed relative to reaction time
In addition, a growth experiment with Arabidopsis (commonly known as Shepherd's purse) was carried out using the products (a mixture of urea and isosorbide) obtained from the degradation of polycarbonate, and it was confirmed that the degradation products obtained from polycarbonate served as fertilizer. Compared to using only commercial urea as fertilizer, Arabidopsis plants grew better when the degradation products, which were a mixture of urea and isosorbide, were used (Fig. 4). The results also suggest that Arabidopsis may grow better by absorbing nitrogen nutrients more efficiently if isosorbide and urea were mixed in the appropriate ratio.
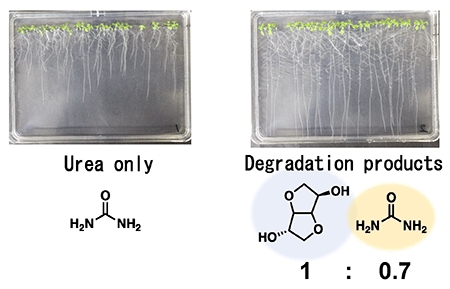
Fig. 4 Arabidopsis growth experiment
Contributing to solving plastic waste and food problems
The production of fertilizer by ammonia-induced degradation of polycarbonate demonstrated in this experiment does not require expensive catalysts and can be performed with simple operations, which has raised great expectations for its industrialization.
By modifying the basic structure of polycarbonate and the way the molecules are linked, it is planned to establish a system whereby plastics can be recycled into fertilizers while expressing desired functions and properties, with the aim of implementing this system in society.
This recycling system not only recycles isosorbide, a biomass resource that is the starting material, but also produces urea that promotes plant growth. According to Fertilizers Europe, more than a century after the invention of ammonia synthesis, the food produced by using nitrogen fertilizers such as urea still feeds 50% of the world population. It is hoped that this recycling system will be elevated to the status an innovative system that simultaneously solves the "plastic waste problem" and the "food problem caused by population growth".
