Research Results
100-year-old mystery: Mechanism of the crystalline lens of the eye becoming transparent revealed
A new intracellular degradation system discovered beyond the framework of autophagy researchFY2022

- Mizushima Noboru (Professor, Graduate School of Medicine, The University of Tokyo)
- ERATO
- "MIZUSHIMA Intracellular Degradation"
Research Director (2017-2022)
Research that unraveled a 100-year-old mystery
In 2016, "Autophagy" (*1) (Fig. 1) became famous when Professor Yoshinori Ohsumi of Tokyo Institute of Technology received the Nobel Prize in Physiology or Medicine. It is one of the intracellular degradation systems of living cells. It has been studied for 60 years or more, but there are still many unknowns. Professor Noboru Mizushima's research project is focusing on autophagy, and is trying to clarify various questions related to intracellular degradation.
So far, Professor Mizushima's group has reported on important new discoveries related to autophagy one after another, including "Progressive reduction of autophagy genes," "Identification of new receptors involved in selective autophagy (*2) of the endoplasmic reticulum (*3)", and "A new role for autophagy - Contributing to the mechanism of inflation of lungs and swim bladders -." In this research project, the group has also been trying to unravel a previously unknown phenomenon different from autophagy. This is the mechanism whereby the crystalline lens (*4) (Fig. 2) of the eye maintains its transparency, which has remained a mystery for 100 years.
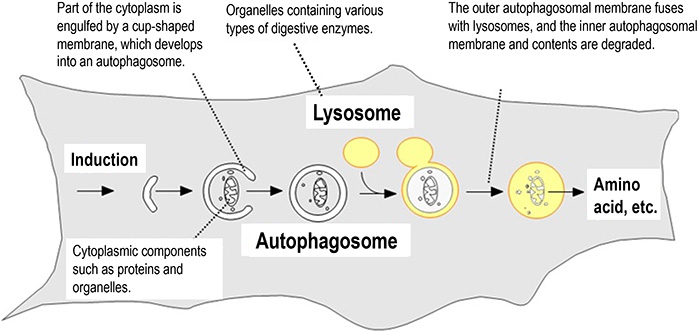
Fig. 1 The mechanism of autophagy
When autophagy is induced, autophagosomes are formed engulfing cytoplasmic components. The autophagosomes then fuse with lysosomes, and the cytoplasmic components enclosed by the autophagosomes are degraded. Degradation products such as amino acids produced by the decomposition of cytoplasmic components are recycled.
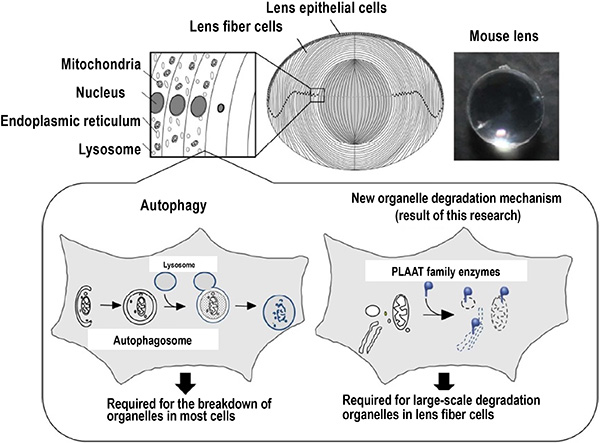
Fig. 2 Schematic diagram of large-scale organelle degradation in the crystalline lens, and overview of the autophagy-independent organelle degradation system discovered in this study
The crystalline lens is composed of epithelial cells and fiber cells. During the final differentiation process of fiber cells , all organelles surrounded by membranes are degraded (above). The group clarified that the degradation of organelles in the crystalline lens is due to the PLAAT family of lipolytic enzymes present in the cytosol. Also revealed was that this new degradation system is important for maintaining the transparency of the crystalline lens (lower right).
*1 autophagy
A common mechanism of eukaryotes, from yeast to humans. It prevents the accumulation of abnormal proteins. And it also degrades and recycles proteins when cells become starved. As a result, it plays a role in maintaining the constant environment in the living body.
*2 selective autophagy
Traditionally, autophagy has been considered a non-selective degradation mechanism. However, recently, it has become clear that there is a mechanism that can selectively identify and degrade damaged organelles, denatured proteins, and intracellular pathogens (selective autophagy).
*3 endoplasmic reticulum
One of the organelles. About one-third of the protein in the cell is synthesized in the endoplasmic reticulum, correctly folded and modified, and secreted. It also has important roles such as storage of ions including calcium, lipid synthesis, and other metabolism and detoxification functions.
*4 crystalline lens
A transparent tissue in the eye. Together with the cornea located on the outside of the crystalline lens, it plays a role in transmitting and refracting light coming from the outside world, focusing it on the retina, and forming an image. In the process of differentiation of lens fiber cells, newly differentiated fiber cells accumulate on the outside of old cells. Therefore, inside the crystalline lens, all the cells made in the past are still present.
*5 Organelles
Structures present inside eukaryotic cells often separated by membranes. They consist of the nucleus, mitochondria, endoplasmic reticulum, Golgi apparatus, lysosomes, peroxisomes, etc., each of which has a different function.
*6 Autophagosome
An organelle that mediates autophagy degradation, and surrounds proteins and organelles present in the cytoplasm (Fig. 1). Autophagosomes fuse with lysosomes, and their contents are digested by degrading enzymes within the lysosomes.
The mystery of the crystalline lens that no one could solve
A crystalline lens acts like a camera lens in the eye. It is a transparent tissue that collects and refracts light coming from the outside world, focuses it on the retina, and forms an image. This allows us to "see".
The cells that make up the crystalline lens are transparent. Organelles (*5) that originally exist, are not present. This is because the cells that make up the crystalline lens break down all organelles during their maturation process. This phenomenon has been known for 100 years or more, but its mechanism was poorly understood. In most cells, organelles are primarily degraded by autophagy. However, Professor Mizushima's group previously showed that the degradation of these organelles that occurs in the cells of the crystalline lens is not due to autophagy. Mizushima et al. predicted that the crystalline lens has a new organelle degradation system different from autophagy. This is large-scale organelle degradation that occurs in the crystalline lens in a short period of time. The detailed mechanism had hardly been explained.
Discovery of a new degradation system different from autophagy
The crystalline lens is composed of epithelial cells and differentiated fiber cells. Organelle degradation occurs at the final stage of differentiation into fiber cells. In this research project, in order to analyze the mechanism of degradation of the organelles of the crystalline lens, the group used the zebrafish as a model, which makes it easy to modify genes, and observe cells and tissues in a living state. First, a zebrafish was produced in which the cell organelles were visualized by a fluorescent protein, and the organelles of the crystalline lens were observed alive. As a result, it was finally revealed how the organelles are actually degraded, and how their contents diffuse into the cytosol (*7) (Fig. 3).
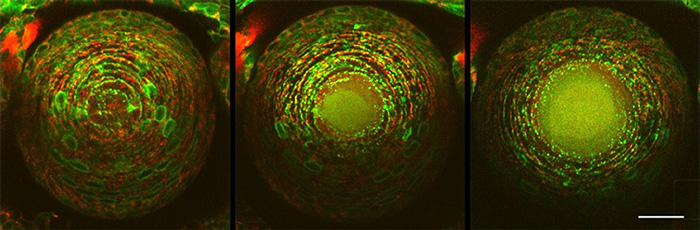
Fig. 3 Progress of organelle degradation in the crystalline lens
Green shows the endoplasmic reticulum, and red shows mitochondria. Looking at the central part of the crystalline lens, the endoplasmic reticulum and mitochondria are degraded and their contents diffuse into the cytosol at 61 hours (middle) and 67 hours (right), compared to 55 hours (left) after fertilization.
The contents of the organelles are dispersed, so the group looked at how the membranes of the organelles are broken down. Since membranes are made of lipids, they proceeded to unravel the mechanism by focusing on lipolytic enzymes. As a result, it was found that Plaat1, which belongs to the enzyme family PLAAT (*8), is required for the degradation of organelles of the crystalline lens.
Plaat1 is normally present in the cytosol and migrates to the membranes shortly before the degradation of organelles (Fig. 5). It was shown that Plaat1 translocates when the mitochondrial or lysosomal membrane is partially damaged. It was also shown that the transcriptional regulator Hsf4 (*9) required for lens development is needed for partial damage to this membrane, and membrane translocation of Plaat1.
*7 Cytosol
Refers to the soluble part of the cytoplasm (the part other than the nucleus in the cell), excluding large structures such as organelles and the cytoskeleton.
*8 Plaat
Abbreviation for Phospholipase A and acyltransferase. Also called HRASLS (HRAS-like suppressor). It is a lipid-metabolizing enzyme that decomposes glycerophospholipids that make up biological membranes, and forms a family consisting of several types in vertebrates. It was found that Plaat1 in zebrafish and PLAAT3 in mice are required for lens organelle degradation.
*9 Hsf4
Abbreviation for Heat shock factor protein 4. A transcriptional regulator that is the causative gene of human hereditary cataract and is highly expressed in the central lens. It is known to induce the genetic expression of some of the crystallins present in high concentrations in the crystalline lens, and DNA-degrading enzymes.
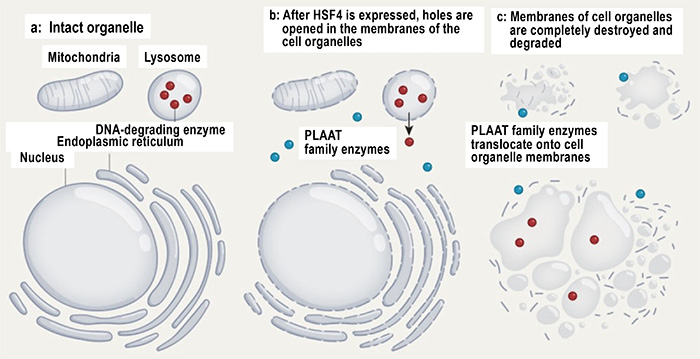
Fig. 4 Degradation of lens organelles
After the expression of transcription factor HSF4, small holes are generated in the membranes of organelles by an unknown mechanism. PLAAT moves to the holes and degrades the membranes. Some DNA-degrading enzymes also leak through small holes in lysosomes and degrade nuclear DNA.
(Source) Boya, P. Nature 592, 509–510 (2021).
The PLAAT family enzymes are widespread in vertebrates, including mammals, and it was found that PLAAT3 is required for organelle degradation of lens cells in mice. Cataract-like symptoms, namely opacity of the crystalline lens and abnormal light refraction, were observed in the crystalline lens of Plaat1-deficient zebrafish and PLAAT3-deficient mice (Fig. 4). It was therefore concluded that large-scale degradation of cellular organelles by PLAAT family enzymes is necessary for crystalline lens transparency.
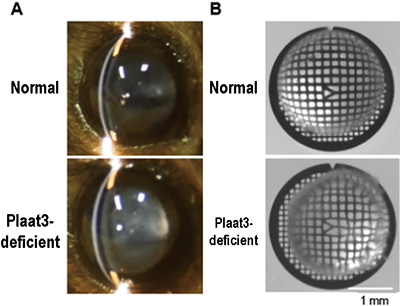
Fig. 5 Plaat3-deficient mouse shows cataract and refractive error
A. External appearance of lens of mouse.
The lens of a Plaat3-deficient mouse shows signs of cataract.
B. Bright-field image of lens of mouse on a grid.
The lens of a Plaat3-deficient mouse shows the distortion of the grid pattern, having refractive errors.
This research was an epoch-making achievement that elucidated one of the important issues remaining in cell biology and developmental biology, "the mechanism and significance of large-scale organelle degradation." PLAAT family enzymes are also expressed in various tissues other than the crystalline lens, and it is considered that they may contribute to the modification of the intracellular environment and the maintenance of homeostasis.
In the future, the detailed molecular mechanism and function of this new organelle degradation system will be clarified, and its difference from and relationship to other intracellular degradation systems such as autophagy will be elucidated, which is expected to lead to a more comprehensive understanding.
- Life Science
- Research Results
- Japanese
