Research Results
Contributing to improvement of fuel cell performance
"Coordination polymer glass" conducts hydrogen ions at high speed without humidificationFY2021
- Denso Corporation /
Kitagawa Susumu (Distinguished Professor,Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University)
Horike Satoshi (Associate Professor, Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University) - Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP)
- Research and Development of Fuel Cells Using Ion-Conductive Coordination Polymer as Electrolyte (2015 - 2020)
New material contributing to popularization and promotion of fuel cells developed.
Fuel cells*1 are promising clean energy sources, and fuel cell cars have already been commercialized. However, technological innovations such as efficiency improvement, reduction of the use of precious metal catalysts, e.g., platinum, and downsizing and weight reduction are necessary to popularize fuel cells. One of the key technologies is a "solid electrolyte*2" that can conduct hydrogen ions at high speed with zero humidity even in an environment of 100°C or higher temperature where water evaporates.
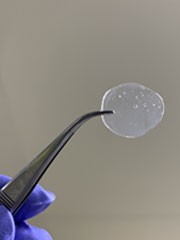
To address this issue, a research group led by Associate Professor Horike Satoshi, in collaboration with Denso Corporation, etc., has successfully synthesized a solid electrolyte material with high hydrogen ion conductivity in an environment of zero humidity and 120°C. The research group has developed a material called "coordination polymer*3 glass," in which metal ions and molecules are alternately linked (Fig. 1). A fuel cell utilizing this material has shown high electrical output performance with a maximum power density of 150 milliwatts per square centimeter for a fuel cell with an electrode area of one square centimeter in an environment of zero humidity and 120°C.
The study results were published on May 13 in "Chemical Science", a journal of the Royal Society of Chemistry in the UK, and was selected as a "ChemSci Pick of the week" as one of the journal's outstanding papers.
*1 Fuel cell
This is a system that uses hydrogen and oxygen gases as fuel to generate electricity. It is considered a clean energy technology because it releases only water upon operation.
*2 Electrolyte
This is one of the materials that constitute a fuel cell, and has the characteristic of being able to transport and conduct hydrogen ions without conducting electricity. Electrolytes made of solids, in particular, are known as solid electrolytes.
*3 Coordination polymer
This is a material in which metal ions and molecules called "ligands" are alternately linked to form a network. Most are crystalline, but some are glass. The structure of those with porosity is called a Metal-Organic Framework (MOF).
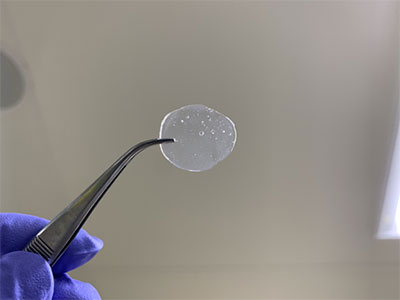
Fig. 1 Coordination polymer glass
This is a "coordination polymer glass" that has been developed as a solid electrolyte material with high hydrogen ion conductivity in an environment of zero humidity at 120°C. Its structure consists of alternating linkages of metal ions and molecules.
Coordination polymer glass attracts attention as electrolyte material for fuel cells that can operate at temperatures of 100°C or higher with zero humidity.
Fuel cells mainly consist of electrodes and an electrolyte. They generate electricity by reforming oxygen and hydrogen with the electrodes and transporting hydrogen ions through the electrolyte. Therefore, the electrolyte material is required to have high hydrogen ion conductivity (Fig. 2).
Although liquid electrolytes show high hydrogen ion conductivity, problems such as deterioration of power generation efficiency and stability due to liquid and gas leaks have been observed. Therefore, solid electrolytes such as organic polymers and ceramics have previously been studied. However, it had been difficult to generate enough electricity because many solid electrolytes could hardly conduct hydrogen ions at high speed in the absence of humidity, and it was difficult to have dense contact with the electrode interfaces. Even though electrolytes that work in an environment of 100°C or higher temperature are required especially for automotive fuel cells, there have been no materials that can conduct hydrogen ions at high speed without water in this temperature range because water, which is the source of hydrogen ions, evaporates in an environment of 100°C or higher temperature.
The research group of Associate Professor Horike and Denso then focused on a material called coordination polymer glass.
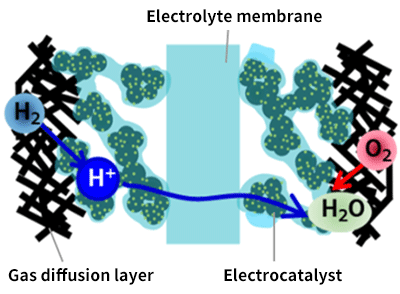
Fig. 2 Illustration of a fuel cell
Fuel cells mainly consist of electrodes and an electrolyte. They generate electricity by reforming oxygen and hydrogen with the electrodes, and transporting hydrogen ions through the electrolyte.
Three-dimensional network structure in which only hydrogen ions can continuously move at high speed.
The research group first used an "ionic liquid*4" consisting of cations and anions for the synthesis of the coordination polymer glass. Some ionic liquids show high hydrogen ion conductivity and form a coordination polymer by reacting with metal ions. In this study, an ionic liquid containing phosphate ions (H2PO4-) from phosphoric acid (H3PO4), which has a large number of hydrogen ions, was used to synthesize the coordination polymer glass in which zinc ions (Zn2+), which are metal ions, are bound to phosphoric acid by adding zinc ions to the liquid (Fig. 3). The resulting coordination polymer glass was highly viscous but soft, and was found to contact well with electrodes. It was also found that it contained a large number of hydrogen ions and showed high hydrogen ion conductivity. Further, the research group performed structural analysis using synchrotron radiation X-ray and solid-state nuclear magnetic resonance (NMR)*5 measurements on this coordination polymer glass. The results show that a wide network of the coordination polymer is three-dimensionally developed inside, and only hydrogen ions can continuously move at high speed due to the dynamic movement of the network (Fig. 4).
The synthesized coordination polymer glass was made into a thin film to examine the power generation characteristics under zero humidity at 120°C, and it was confirmed that it has high hydrogen ion conductivity (Fig. 5).
*4 Ionic liquid
This is a substance that is liquid at room temperature, even though it is a salt consisting only of cations and anions. It has characteristics such as non-volatility and non-flammability.
*5 Solid-state nuclear magnetic resonance (NMR)
This is one of the analyzers. It uses the magnetic energy of atomic nuclei to nondestructively analyze the molecular structure of solid materials at nanoscale dimensions.
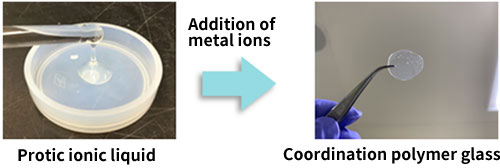
Fig. 3 Synthesis of coordination polymer glass
An ionic liquid (left) containing phosphate ions (H2PO4-) from phosphoric acid (H3PO4), which has a large number of hydrogen ions (protons), was used to synthesize the coordination polymer glass (right) in which zinc ions (Zn2+), which are metal ions, are bound to phosphoric acid by adding zinc ions to the liquid (Fig. 3).
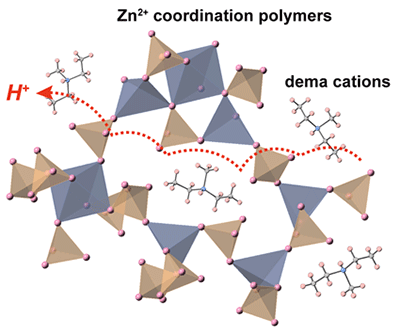
Fig. 4 Illustration of the coordination polymer glass conducting hydrogen ions at high speed.
A wide network of the coordination polymer is three-dimensionally developed, and only hydrogen ions can continuously move at high speed due to the dynamic movement of the network.
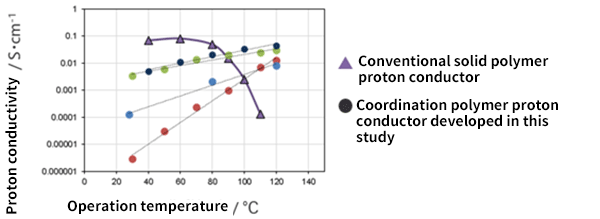
Fig. 5 Conductivity of hydrogen ions
Power generation characteristics under zero humidity at 120°C showed high hydrogen ion conductivity.
The structure can be variously arranged by changing the combination of metal ions and molecules.
The coordination polymer glass synthesized in this study not only shows high hydrogen ion conductivity in an environment of 100°C or higher temperature with zero humidity, but also has an advantage as a solid electrolyte in that it is softer than crystals because it is glass, and can be formed into desired shapes such as membranes and fibers by heating. In addition, the structure of the coordination polymer glass can be variously arranged by changing the combination of metal ions and molecules, and its softness can also be adjusted depending on the combination. The research group plans to further improve fuel cell performance by studying a wider range of metal ions and molecules for forming the glass, aiming for practical use.
- Environment and Energy
- Research Results
- Japanese
