Research Results
Results that will contribute to the promotion of basic research in general cell biology as well
Key mechanism regulating autophagy elucidatedFY2021

- Noda N. Nobuo (Laboratory Head, Laboratory of Structural Biology, Institute of Microbial Chemistry)
- CREST
- [Structural Life] Research Director of the project, "Structural Life Science for Elucidation of Membrane Dynamics of Autophagy" in the research area, "Structural Life Science and Advanced Core Technologies for Innovative Life Science Research" (2013 - 2020)
Mechanism and nature of structures responsible for function of autophagy elucidated.
Autophagy is a degradation process of proteins in cells during a state of nutrient starvation. It is a basic and primary function found in almost all eukaryotes, from yeasts to humans. Since abnormalities in autophagy cause neurodegenerative diseases and cancer, understanding its mechanism is also essential for the development of treatments and prevention of diseases. However, the mechanism and nature of the structures responsible for autophagy in a cell have long been unknown. Little is known about the mechanism of autophagy, in particular, how autophagosomes, which are pouch-like lipid membranes holding degradation targets, expand.
To address this issue, Noda's group in CREST has elucidated that the structure responsible for autophagy is a liquid droplet*1formed by "liquid-liquid phase separation*2" of the Atg proteins (Fig. 1). This is a new discovery that shows that the phenomenon of liquid-liquid phase separation directly regulates autophagy. Noda et al. also found that the membrane protein Atg9 acts in the expansion of autophagosomes.
New drugs that regulate autophagy are expected to be developed in the future.
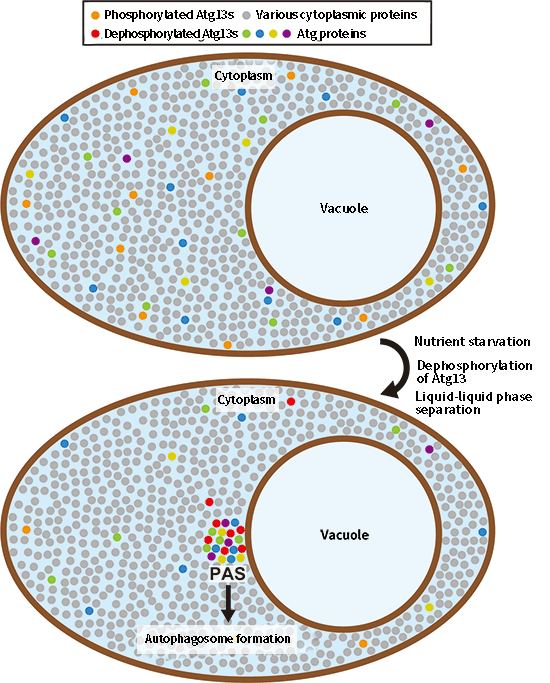
Fig. 1 The structure responsible for autophagy is a liquid droplet formed by "liquid-liquid phase separation",
Atg proteins are dispersed in the cytoplasm under nutrient-rich conditions, whereas nutrient starvation and dephosphorylation of Atg13 causes Atg13, along with other Atg proteins, to "liquid-liquid phase separate" and form a new liquid phase (droplet) on the vacuolar membrane. Autophagosomes are formed from those droplets.
*1 Liquid droplet
This is a liquid-like aggregate formed by liquid-liquid phase separation of proteins and/or nucleic acids. Droplets, also known as "membraneless organelles", perform various functions in a cell. Droplets spontaneously form spheres, have high internal fluidity, and actively exchange molecules with their surroundings.
*2 Liquid-liquid phase separation
This is a phenomenon in which a homogeneous solution separates into two or more liquid phases. While there are organelles separated by membranes such as the nucleus and mitochondria in a cell, nucleic acid and protein droplets have recently been considered to play various roles as "membraneless organelles". Membraneless organelles are likely to have various roles, as they can form and dissolve quickly in response to environmental changes.
Droplets formed by liquid-liquid phase separation of Atg proteins regulate autophagy.
In autophagy, when the cell is nutrient-starved, a pouch-like lipid membrane called an autophagosome is created, and it holds degradation targets. They are then transported to a lysosome where degradation takes place. It was known that about 20 different Atg proteins were involved in the production of autophagosomes. It was also known that the Atg proteins aggregate to form a structure called PAS*3. However, the mechanism of how Atg proteins aggregate and the nature of the PAS formed had not been well understood.
To address this issue, Noda's group used fluorescence microscopy to examine Atg13, a type of Atg protein, in detail. They further reconstructed PAS in vitro to study its behavior. They also analyzed PAS using a high-speed atomic force microscope*4.
The results showed that PAS is a droplet formed by the liquid-liquid phase separation of Atg13 along with other Atg proteins, and that this droplet concentrates on the vacuolar membrane to form PAS, forming autophagosomes.
*3 PAS
PAS stands for "pre-autophagosomal structure", Atg proteins aggregate in a single location near the vacuole during nutrient starvation in yeast. PAS refers to this aggregated structure. Autophagosomes are presumably formed with PAS as a starting point.
*4 High-speed atomic force microscope
An atomic force microscope (AFM) is a microscope that visualizes the shape of molecules based on the atomic forces acting between the tip and the sample. Among them, a high-speed AFM is an instrument that can observe biomolecules such as proteins moving in solution with nanometer-scale spatial resolution and sub-second-scale time resolution.
Atg9 causes expansion of autophagosome membrane.
The most important feature of autophagy is that it creates new autophagosomes. The process of forming autophagosomes determines the degradation targets. Approximately 20 types of Atg proteins are involved in the formation of autophagosomes, most of which have been analyzed for structure and function. Noda's group previously discovered that Atg2, a lipid transfer protein, carries phospholipids from endoplasmic reticula to form autophagosomes. However, how the lipid membrane of the autophagosome expands using the transported phospholipids was still completely unknown (Fig. 2).
They focused on Atg9, the only membrane protein whose function was unknown among the Atg proteins involved in autophagy. Using Atg9 extracted from yeast, they carefully examined its function in vitro. As a result, they discovered that Atg9 works to move the lipids that constitute the membrane of autophagosomes from one layer to the other of the "lipid bilayer" forming a liposome. The three-dimensional structure of yeast Atg9 examined by cryo-electron microscopy revealed that Atg9 has pores connecting the two layers of lipid bilayer (Fig. 3).
These findings indicate that Atg9 causes the expansion of autophagosome membranes. This means that Atg9 cooperates with Atg2 in the formation of autophagosome membranes (Fig. 4). This discovery is a new mechanism that has not previously been predicted, and is a breakthrough that will change the direction of basic research on autophagy.
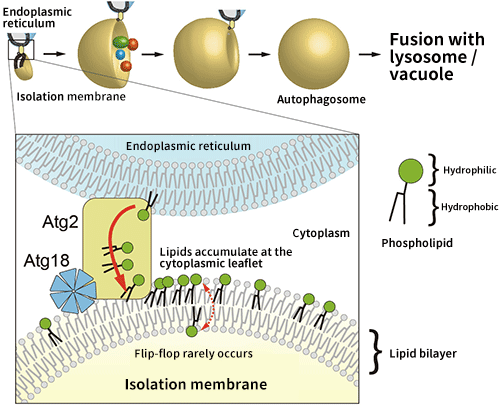
Fig. 2 Schematic diagram of the autophagosome formation process
Induction of autophagy results in the sudden appearance of a membrane structure in the cytoplasm (this is called an isolation membrane), which expands while enclosing the degradation targets, and then closes to form an autophagosome. Atg2 may supply phospholipids from an endoplasmic reticulum, which are necessary for the isolation membrane to expand, but lipids accumulate at the cytoplasmic leaflet of the isolation membrane, preventing the membrane from expanding.
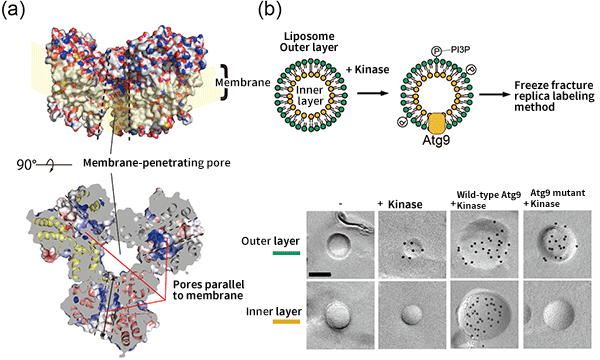
Fig. 3 Structure of Atg9 and its function
(a) Atg9 has a membrane-penetrating pore at its center and pores parallel to the membrane in each molecule, which link to form channels for lipids. (b) Atg9 has a function to transfer lipids (PI3P), which constitute an autophagosome membrane, from the outer layer to the inner layer of a liposome. However, the activity is suppressed by pore mutants. The black dots represent the PI3P distribution.
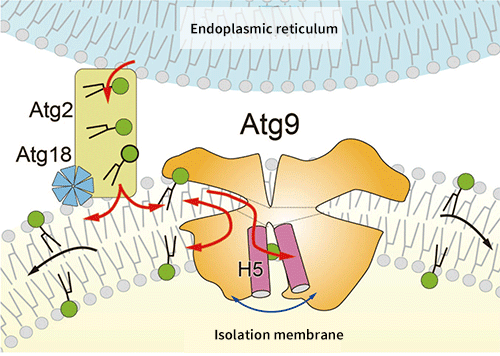
Fig. 4 Expansion mechanism of isolation membrane by Atg9 and Atg2
Atg2 connects the endoplasmic reticulum membrane to the isolation membrane, and transfers phospholipids contained in the endoplasmic reticulum to the cytoplasmic leaflet of the isolation membrane. Atg9, as well as Atg2 and Atg18, is localized at the tip of the isolation membrane, and expands the isolation membrane by transferring phospholipids transported by Atg2 to the opposite layer.
Results contribute to promotion of basic research in cell biology in general.
The discovery of the direct regulation of autophagy by liquid-liquid phase separation is an achievement that once again brings attention to the broad involvement of liquid-liquid phase separation in all biological phenomena in a cell. The elucidation of the mechanism of autophagosome membrane expansion, which has been unknown for a long time in autophagy, is also expected to help to rapidly clarify the extremely complex mechanism of autophagy in the future. The elucidation of the mechanism will promote research for the treatment and prevention of various diseases related to autophagy. Further, the mechanism of membrane expansion by the cooperation of lipid transfer proteins and lipid scramblases discovered in this study is the first to be revealed not only in autophagy but also in cell biology, and is expected to contribute to the promotion of basic research in cell biology in general.
- Life Science
- Research Results
- Japanese
