Research Results
Analyze three-dimensional structure of macromolecules in vivo
Develop a new method using cryo-electron microscopeFY2020

- JEOL Ltd./Fujiyoshi Yoshinori(Distinguished Professor Tokyo Medical and Dental University)
- NexTEP(Newly extended TEchnology transfer Program)
- “Real environment high resolution 3D biostructural analysis system”
Development company/Representative researcher(2015-2019)
Realize three-dimensional structural analysis for macromolecules in vivo at a resolution of the world’s highest level by applying a new method to the latest cryo-electron microscope
The three-dimensional structure of macromolecules in vivo such as protein is the basic information that is essential to the research on life science and drug discovery. In recent years, examples of structural analysis of protein using a cryo-electron microscope (electron microscope at low temperature) has rapidly increased. JEOL Ltd. (the development company of NexTEP) and Professor Fujiyoshi Yoshinori (principal investigator of the project) were the first to develop a cryo-electron microscope in Japan, and they have been improved it over thirty years. In the latest instrument, a new technology to analyze the structure of protein in lipid membrane, which had been difficult with conventional instruments, is equipped. The new instrument has attracted increasing attention, because it is expected to be able to analyze the three-dimensional structure of proteins and drugs at a resolution of the world’s highest level.

Fig. 1 Latest cryo-electron microscope equipped with new technology by NexTEP.
Elucidate three-dimensional structure of membrane protein called “water channel” by electron beam crystallography.
Cryo-electron microscopes are characteristic in that the macromolecules in vivo can be analyzed without exposure to air and radiation damage by the electron beam can be reduced, by rapidly cooling the sample. The 2017 Nobel Prize in Chemistry was awarded to three researchers in Europe and the US who invented the basic technology of cryo-electron microscopy. However, the JEOL Ltd. and Professor Fujiyoshi have also been collaboratively developing the original instrument in Japan since the dawn of the late 1970s. In 1986, they succeeded in developing the first instrument in the world in which the radiation damage by electron beam was minimized.
One of the important results of the cryo-electron microscope developed by JEOL Ltd. and Professor Fujiyoshi in collaboration was the structural analysis of membrane protein called “water channel” using electron beam crystallography. About 70% of the human body is composed of water. Therefore, in order to keep life and brain functions healthy, water channels that allow only water molecules to pass play an important role. In a human body, thirteen kinds of water channels exist, i.e., from aquaporin 0 to aquaporin 12. In particular, aquaporin 1, which is found in many tissues, allows up to three billion water molecules in a second to pass, but does not allow any ions or protons to pass. In order to clarify this mysterious mechanism, Professor Fujiyoshi analyzed the three-dimensional structure of aquaporin 1 for the first time in the world using the cryo-electron microscope developed in collaboration with JEOL Ltd., by making full use of electron beam crystallography. As a result, he has elucidated that only water molecules specifically pass the water channels at high speed depending on the direction of hydrogen in water molecules.
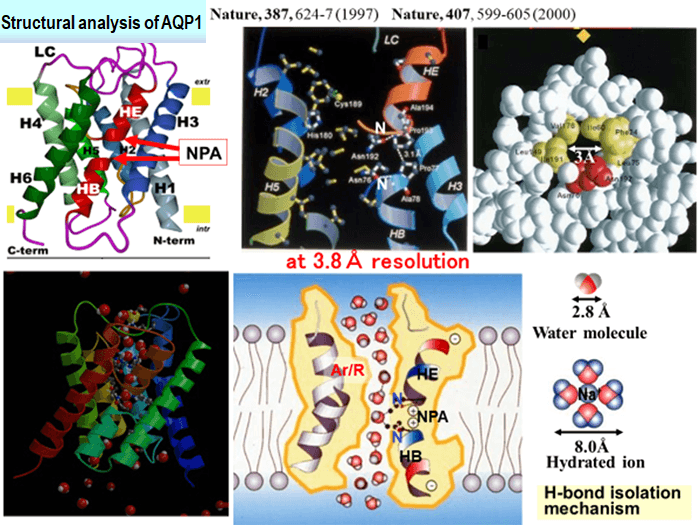
Fig. 2 Analytical results of three-dimensional structure for aquaporin 1.
Realization new method for three-dimensional analysis in vivo, “IBSA method”.
In electron beam crystallography, in order to analyze the structure of a membrane protein in a lipid membrane, it had been required to extract and crystallize the sample from the lipid membrane. On the other hand, membrane proteins such as water channels can demonstrate their functions only when they are inside lipid membranes. Therefore, taking this property into consideration, Professor Fujiyoshi newly invented a method for structural analysis called “IBSA (Image Based Structure Analysis).” In the IBSA method, an image of a macromolecule in vivo is first taken at a high spatial resolution under tilted conditions. Then, the same field of view is taken also under non-tilted conditions. By referencing the non-tilted image, the three-dimensional structure is constructed from the initially taken tilted image.
Based on this idea, JEOL Ltd. and Professor Fujiyoshi have developed a cryo-electron microscope equipped with the technology of the IBSA method at NexTEP. As to the role-sharing, JEOL Ltd. has taken charge of developing a cryo-electron microscope, and Professor Fujiyoshi has developed software to collect data on the IBSA method and software for analysis. As a result, for β-galactosidase*1, one of the enzymes, a spatial resolution of 0.27 nm could be achieved based on the evaluation by the FSC (Fourier Shell Correlation) method※2 (FSC=0.143). In May 2019, the result was certified as a successful NexTEP development project.
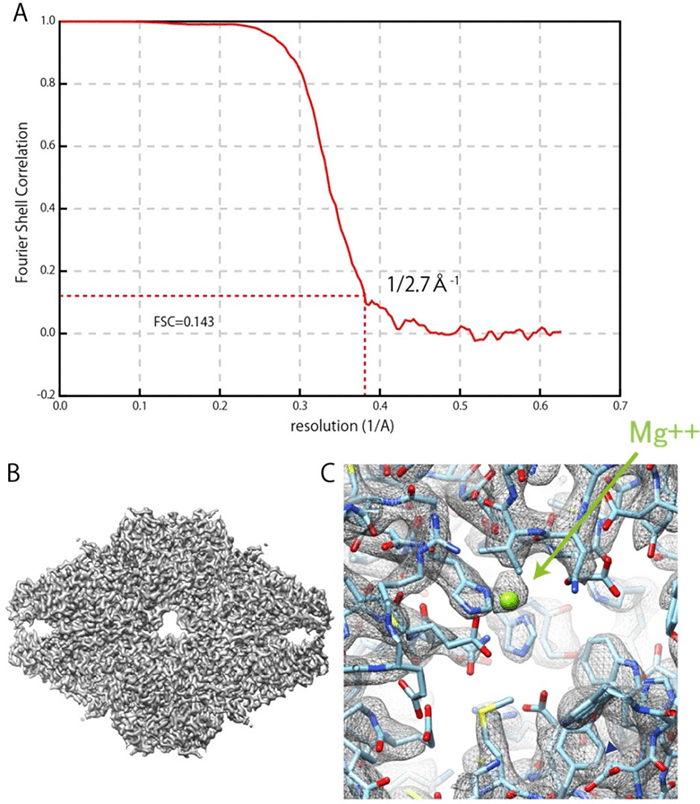
Fig. 3 Results for three-dimensional reconstruction of β-galactosidase
(A) FSC plot. The red dotted line indicates FSC=0.143. A spatial resolution of 0.27 nm was defined from the first intersection between the red dotted and solid lines.
(B) Map of three-dimensional reconstruction, and atomic model.
(C) Magnesium ions at the active part in enzyme.
*1β-galactosidase
Protein originating from escherichia coli. It is an enzyme that functions in the form of tetramer and decomposed lactose into glucose and galactose. Its structure has been investigated by X-ray structure analysis for a long time.
*2 FSC (Fourier Shell Correlation) method
One of the methods for estimating spatial resolution. Particles selected from electron microscope images for the analysis are randomly divided into two groups in advance. Then, a three-dimensional structure is reconstructed independently in each group. By performing the Fourier transformation for the obtained independent three-dimensional structures, the correlation between two groups at each region at arbitral spatial resolution is calculated. In many cases, the resolution position where the correlation value FSC reaches 0.143 for the first time is defined as the resolution of the analysis result.
Remote operation system enabled the use of a latest cryo-electron microscope
In April 2017, Professor Fujiyoshi and JEOL Ltd. established a company called CeSPIA (Chiyoda-ku, Tokyo) with a joint investment. The company is engaged in the entrusting and consulting of three-dimensional structural analysis for drug discovery. When a customer delivers samples to CeSPIA, the results for the structural analysis by a cryo-electron microscope at high spatial resolution can be obtained at an affordable price.
Furthermore, the latest cryo-electron microscopes developed at this time are now installed in JEOL Ltd. It can be operated by a remote-control system from a laboratory in the Tokyo Medical and Dental University while looking at the monitor display. Since cryo-electron microscopes are expensive, costing hundreds of million yen per machine, and difficult to maintain, JEOL Ltd. is planning to open cryo-electron microscopes for public use for companies and research institutes with insufficient funds, by introducing a remote-control system.
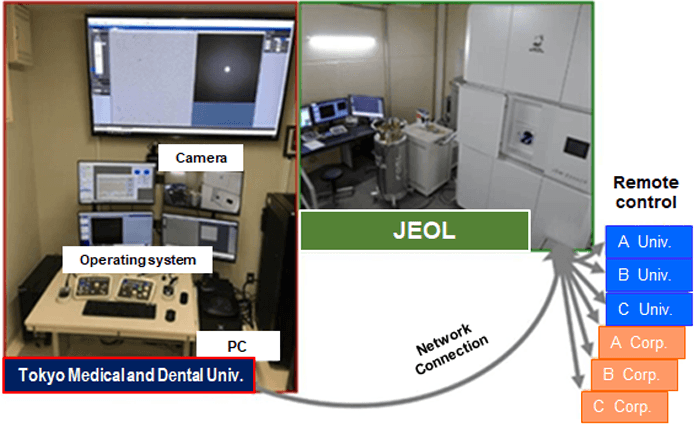
Fig. 4 Plan to open cryo-electron microscope to public used by remote-control system.
- Life Science
- Research Results
- Japanese
