Research Results
Just mix in flask
Innovative “Method of Ammonia Synthesis”FY2020

- Nishibayashi Yoshiaki(Professor, Graduate School of Engineering, The University of Tokyo)
- CREST
- Creation of Innovative Core Technology for Manufacture and Use of Energy Carriers from Renewable Energy「Development of innovative methods for ammonia production and related reactions by using transition metal catalysts」Research Director(2015-2020)
Develop new method of ammonia synthesis alternative to the Haber-Bosch method
Ammonia (NH3) is widely used for raw materials such as nitrogen fertilizers, nylon fibers for clothes, and medicines. In addition, since ammonia does not generate carbon dioxide (CO2), which is a greenhouse gas even when burned, easily becomes liquid, and is easy to store and transport, it is expected to be used as an energy source for thermal power generation.
At present, ammonia is synthesized by a chemical reaction between nitrogen (N2) and hydrogen (H2) at factories by the “Haber-Bosch method.” In the Haber-Bosch method, however, high temperatures (400-600℃) and high pressures (100-200 atm) are required for the chemical reaction between nitrogen and hydrogen. Furthermore, a large amount of energy is required and a large amount of CO2 is generated through the process, because hydrogen is now produced mainly from fossil fuels such as oil, coal and natural gas. Therefore, a new method of ammonia synthesis that replaces the Haber-Bosch method has been strongly expected to be developed.
Under such circumstances, Professor Nishibayashi Yoshiaki, Research Director of CREST, succeeded in developing an innovative method to simply and rapidly synthesize large amounts of ammonia from nitrogen gas and water at room temperature and atmospheric pressure for the first time in the world. The research result, published in Nature (online) in April 2019, is now attracted worldwide attention.
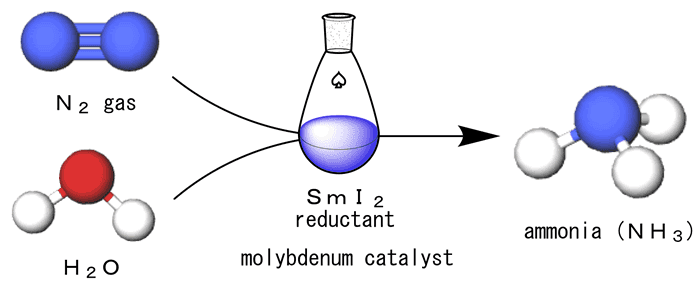
Fig. 1 Development of an innovative method to simply and rapidly synthesize significant amounts of ammonia from nitrogen gas and water at room temperature and atmospheric pressure.
Develop innovative method of synthesis getting the hint from “rhizobia” that grows symbiotically with leguminous plants
In the Haber-Bosch method, fossil fuels are used for producing hydrogen that is the raw material of ammonia. In nature, however, it is known that there is an enzyme that synthesizes ammonia at room temperature and atmospheric pressure. It is “nitrogenase” in bacteria named “rhizobia” that grows symbiotically with leguminous plants possesses. When nitrogenase synthesizes ammonia from nitrogen, it uses molybdenum (Mo) existing in the nitrogenase molecule. Focusing on this nitrogenase, Professor Nishibayashi succeeded in synthesizing ammonia at room temperature and atmospheric pressure at a speed comparable to that by nitrogenase, by using samarium diiodide (SmI2).
The more detailed reaction process is as follows. First, a nitrogen molecule (N2) is bonded to a catalyst containing molybdenum. Next, another catalyst approaches, and then breaks the chemical bond between two nitrogen atoms (N) in the nitrogen molecule. As a result, the nitrogen atom (N) is bonded to the catalyst containing molybdenum. Then, samarium diiodide approaches there. Samarium diiodide plays a role as a reducing reagent that produces hydrogen (H), a raw material of an ammonia molecule (NH3), from a water molecule (H2O). Three hydrogen atoms produced by the samarium diiodide and water are bonded to the nitrogen atom, forming ammonia. The produced ammonia is separated from the catalyst, then the catalyst returns to its original state. By repeating this cycle, a large amount of ammonia is synthesized.
It can be said that this synthesis process is “an artificial reproduction of the enzyme mechanism of nitrogenase.”
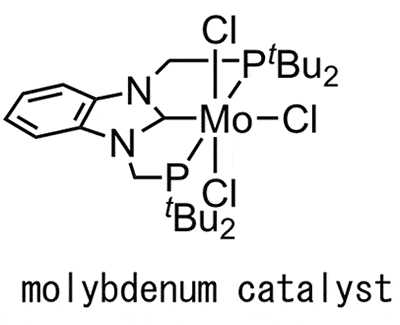
Fig. 2 Structure of catalyst containing molybdenum.
Application of samarium diiodide became breakthrough
The advantage of this synthesis method is that is excellent both in cost and energy. Hydrogen can be produced at room temperature and atmospheric pressure. In addition, the method scarcely needs energy to synthesize ammonia. Ammonia can be simply synthesized by mixing it in a flask.
One of the important points that this synthesis method made possible was that Professor Nishibayashi discovered a phenomenon wherein ammonia synthesis proceeds at room temperature and atmospheric pressure at a speed comparable to nitrogenase by using samarium diiodide. The combination of samarium diiodide and water has been used in organic synthesis for a long time as a reducing reagent for the carbon-oxygen double bond (C=O). The great breakthrough was that Professor Nishibayashi noticed that this reagent could be used also for the reduction of the nitrogen-nitrogen triple bond (N≡N).

Fig. 3 Nitrogen and water are put into the mixture of molybdenum-containing a catalyst and reducing reagent (samarium diiodide) at room temperature and atmospheric pressure. Then, only by mixing in flask, ammonia is synthesized.
Expected to solve environmental and energy problems
Although samarium (Sm) is inexpensive, it has to be reused when it is practically applied, because it is one of the rare-earth elements. Focusing on the practical application of samarium, the research group of Professor Nishibayashi plans to work on the industrialization of collection and reuse cycle of samarium in collaboration with industries.
Furthermore, the present research results not only invented the innovative method of ammonia synthesis, but are also expected to greatly contribute to the future resolution of environmental and energy problems. Hydrogen is currently attracting much attention as a next-generation energy source. However, there remains the problem that a large amount of CO2 is generated to produce hydrogen. Also, the method to store and transport hydrogen is another problem to be solved. As to these problems, ammonia has advantages, because it easily becomes liquid and the storage/transport of ammonia is easy. In addition, since ammonia does not generate CO2 even when burnt like hydrogen, it can be used for fuels of thermal power generation as a clean energy source. This synthesis method has the potential to cause a paradigm shift in the energy resources of society.
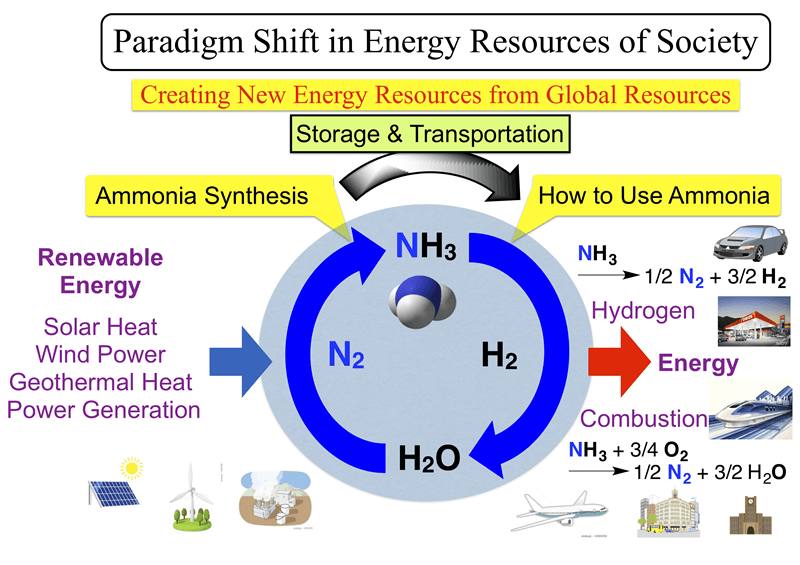
Fig. 4 The results not only invented the innovative method of ammonia synthesis, but also have the potential to possibly cause a paradigm shift in the energy resources of society.
- Environment and Energy
- Research Results
- Japanese
