Proteoliposome engineering
Liposome chaperon in cell-free membrane protein synthesis: One-step preparation of KcsA-integrated liposomes and electrophysiological analysis by the planar bilayer method
| Chaperoning functions of liposomes were investigated using cell-free membrane protein synthesis. KcsA potassium channel-reconstituted liposomes were prepared directly using cell-free protein synthesis. In the absence of liposomes, all synthesized KcsA protein aggregated. In the presence of liposomes, however, synthesized KcsA spontaneously integrated into the liposome membrane. The KscA-reconstituted liposomes were transferred to the planar bilayer across a small hole in a thin plastic sheet and the channel function of KcsA was examined. The original electrophysiological activities, such as voltage- and pH-dependence, were observed. These results suggested that in cell-free membrane protein synthesis, liposomes act as chaperones, preventing aggregation and assisting in folding and tetrameric formation, thereby allowing full channel activity. | 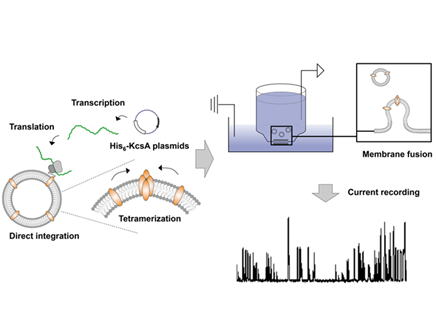 Fig.1 Preparation of KcsA-integrated proteoliposomes by using cell-free membrane protein synthesis / liposome system Expressing KcsA spontaneously integrated into liposomal membrane, subsequently KcsA was folded and organized the tetrameric formation. The original electrophysiological activities, such as voltage- and pH-dependence, were observed by using planar bilayer methods. |
| Paper M. Ando, M. Akiyama, D. Okuno, M. Hirano, T. Ide, S. Sawada, Y. Sasaki, K. Akiyoshi, Liposome chaperon in cell-free membrane protein synthesis: one-step preparation of KcsA-integrated liposomes and electrophysiological analysis by the planar bilayer method, Biomater Sci-Uk (2016). |
Comprehensive study of liposomeassisted synthesis of membrane proteins using a reconstituted cell-free translation system
| Structural and functional analysis of membrane proteins is often hampered by their irreversible aggregation in hydrophilic conditions. Previous reports have suggested that protein aggregation could be prevented by including exogenous liposomes in cell-free translation processes. Systematic studies that identify which membrane proteins can be rescued from irreversible aggregation during translation by liposomes would be valuable in terms of understanding the effects of liposomes and developing applications for membrane protein engineering in the context of pharmaceutical science and nanodevice development. Therefore, we performed a comprehensive study to evaluate the effects of liposomes on 85 aggregation-prone membrane proteins from Escherichia coli by using a reconstituted, chemically defined cell-free translation system. The liposomes improved the yields of newly synthesized proteins and reduced protein aggregation. Bioinformatics analyses suggested that the solubilization effects of the liposomes could be predicted from the physicochemical properties of the individual membrane proteins. | 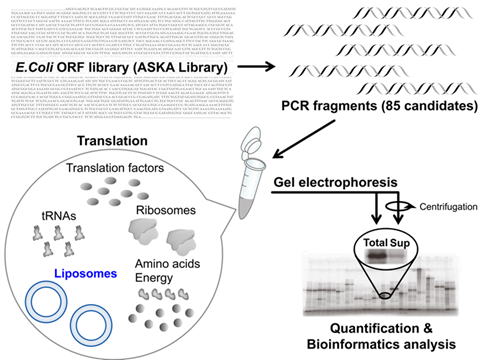 Fig.2 Comprehensive analysis of effects of liposomes on solubilities of membrane proteins The effects of liposomes on 85 aggregation-prone membrane proteins from Escherichia coli by using cell-free translation / liposomes system. Bioinformatics analyses suggested that the solubilization effects of the liposomes could be predicted from the physicochemical properties of the individual membrane proteins. |
| Paper T. Niwa, Y. Sasaki,E. Uemura, S. Nakamura, M. Akiyama, M. Ando,S. Sawada, S. Mukai,T. Ueda, H. Taguchi, and K. Akiyoshi, Comprehensive study of liposomeassisted synthesis of membrane proteins using a reconstituted cell-free translation system、Scientific Reports, in press, Doi:10.1038/srep18025 (2015) |
