Research Results
Adhesion to Hard-to-Bond Materials and Underwater Adhesion Made Possible
Development of a Recyclable Photoreactive AdhesiveFY2024

- NAITO Masanobu (Deputy Director, Research Center for Macromolecules and Biomaterials, National Institute for Materials Science)
- CREST
- Research Director (2019-2024) of the project “Data-centric Molecular Design for Development of Supercomposite Materials”
Development of a recyclable adhesive
The research team led by Deputy Director Masanobu Naito has developed a recyclable adhesive that allows for repeated bonding and debonding in response to light of specific wavelengths.
Traditional adhesives are unable to provide both strong adhesion and easy debonding. They are also hard to recycle as raw materials because strong adhesion often causes issues such as damage to the substrate (the base material bonded) or adhesive residue on the substrate, making it difficult to separate and recover adhesive from the substrate after use.
An adhesive bond by a crosslinking reaction in which the molecules constituting it are connected by a chemical reaction. Debonding utilizes a reverse process called a de-crosslinking reaction. The research team focused on caffeic acid, which can reversibly induce crosslinking and de-crosslinking reactions by irradiation with ultraviolet (UV) light of different wavelengths. The team applied a polymer containing caffeic acid to a substrate and achieved strong adhesion while enabling debonding upon exposure to UV light when finished with use as an adhesive. Both the adhesive and the substrate were recovered for reuse in a state identical to that of the state prior to application (Fig. 1).
This adhesive is expected to be applied to substrates and environmental conditions that are challenging for conventional adhesives, such as fluoropolymers and underwater adhesion.
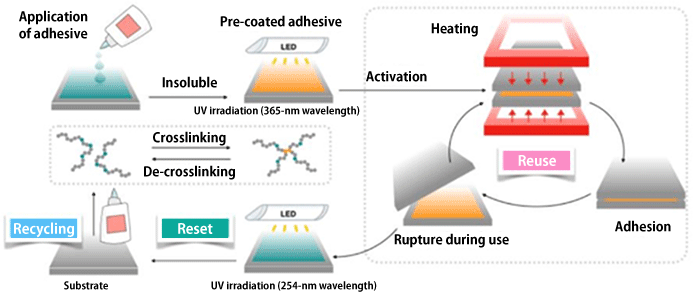
Fig. 1 Reuse and end-of-life recycling of the adhesive
The adhesive can be reused repeatedly even if it ruptures during use, and can be reset and recycled once its purpose has been fulfilled.
Environmental consciousness drawing attention to dismantlable adhesives
During the Industrial Revolution in the UK in the 19th century, the invention of postage stamp gum, which was made mainly of starch and designed to regain adhesiveness when moistened, was a major factor paving the way to the development of the postal system. Since then, pre-coated adhesives,*1 which are applied to substrates in advance to form an adhesive layer, have found wide applications in various fields for their ability to increase workability as well as for their usefulness in inventory management and transportation.
Today, vigorous research and development is underway to search for new bonding technologies, taking into account diverse external stimuli beyond moisture such as electricity, heat, and light.
Meanwhile, as social awareness of the need to pursue both environmental conservation and economic growth has increased, dismantlable adhesives are attracting attention as a bonding technology for manufacturing in the era of the circular economy. Dismantlable adhesives are adhesives that can debond after their service life ends. This technology is needed to realize plastic resource circulation,*2 which is the concept of reusing and recycling end-of-life products containing plastic as new materials by disassembling them into components and then sorting and recovering them by material type.
However, conventional adhesives are usually unable to provide both strong adhesion and easy debonding. Even where debonding is possible, there are problems that hinder material circulation such as adhesive residue on the substrate or damage to the substrate. Adhesives are also unusable on plastics with low adhesiveness, such as fluoropolymers and polyethylene, and unsuitable for certain environments, notably those underwater.
Thus, a new bonding method that demonstrates sufficient adhesive strength for use on any material in any environment and that is easy to debond once its original purpose has been fulfilled is called for.
*1 Pre-coated adhesive
A type of adhesive that is applied onto a
surface before actual use so that it can be activated by an external stimulation and become
adhesive. A typical example is the water-activated adhesive applied to the backs of postage
stamps.
*2 Plastic resource circulation
An initiative aimed at establishing a system for properly treating waste plastic and
effectively using it as a resource.
Confirmation of bonding and debonding by irradiation with UV light of different wavelengths
The research team focused on a function characteristic to caffeic acid, namely that it can reversibly induce crosslinking and de-crosslinking reactions by irradiation with UV light of different wavelengths (Fig. 2). Caffeic acid, also known as coffee acid, is found in coffee and other plants in general.
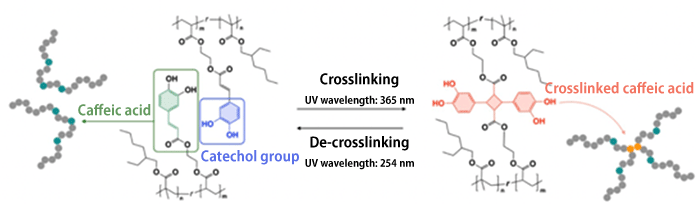
Fig. 2 Scientific structure of pre-coated adhesive containing caffeic acid and its crosslinking/de-crosslinking reactions
A crosslinking reaction occurs when a catechol group contained in caffeic acid is exposed to UV light having a wavelength of 365 nm. In contrast, upon exposure to UV light with a wavelength of 254 nm, the original straight-chain polymer is restored through a de-crosslinking reaction.
For this research, the team synthesized a pre-coated adhesive by introducing caffeic acid units into polymethacrylic acid ester, which is typically used in commercially available adhesives. When a substrate coated with this adhesive material is irradiated with UV light of a specific wavelength, a crosslinking reaction occurs to form an insoluble film. This film is not adhesive at room temperature but can be repeatedly bonded and debonded when heated. The team also found that performance did not deteriorate after the adhesive was stored in a dark place at room temperature for about two years.
Furthermore, when the film is exposed to UV light of a wavelength different than that used for film formation, the caffeic acid returns to the state prior to application. This allows for the complete removal of the adhesive layer remaining on the substrate surface and the recovery of the adhesive itself. The recovered adhesive and substrate can be reused just as they were originally.
Although there have been studies that demonstrated bonding and debonding activated by light, they are applicable only to transparent substrates (e.g., glass substrates). In this research, light is applied from above the coated surface, thereby eliminating such substrate constraints. The team successfully achieved strong adhesion of substrates made of materials such as fluoropolymers, silicone resins, polyethylene, and aluminum (Fig. 3).

Fig. 3 Strong adhesion demonstrated on materials to which conventional adhesives do not bond well
(1) Bonding fluoropolymer material.
(2) Bonded silicone tube and its flow test.
The team also noted the fact that the catechol groups present in the chemical structure of caffeic acid are commonly found in the byssus*3 of mussels, a periphyton species. Under remote operation, the adhesive demonstrated strong adhesion even underwater, a challenging environment for conventional adhesives (Fig. 4).
*3 Byssus
Protein filaments that bivalves such as mussels secrete in order to strongly anchor
themselves to surfaces such as rock reefs or underwater structures.

Fig. 4 Remote operation of underwater adhesion using UV light application and induction heating
(1) Application of an adhesive containing magnetic nanoparticles to a
polypropylene substrate.
(2) UV light application for pre-coating.
(3) Induction heating to bond the substrate.
Wide application to manufacturing in the era of the circular economy
In the era of the circular economy, manufacturers are required to offer products that can be used repeatedly and to recycle products into structural materials by restoring them to their original states as raw materials.
A recyclable adhesive whose reuse and resetting can be managed by applying UV light, without the need for a complex process, is expected to find applications in various fields and to contribute to the advancement of recycling. Furthermore, with the ability to bond in humid environments and underwater, such an adhesive may be used for medical purposes, particularly in vivo applications during surgery, and for remotely operated repair work on offshore structures.
The team also considers this adhesive to be conducive to manufacturing that supports material circulation, and sees future opportunities in various applications, such as electronic devices and transport equipment.
Consultations regarding commercialization have already begun with businesses, with the aim of providing samples within a year.
*Reproduced from the original paper (CC BY 4.0)
