Research Results
Next generation all-solid-state batteries
Sulfide-based solid electrolytes and electrode materialsFY2019

- Masahiro Tatsumisago(President, Osaka Prefecture University)
- CREST
- Creation of Innovative Technologies to Control Carbon Dioxide Emissions “Fabrication of All-Solid-State Rechargeable Batteries with Controlled Solid Interfaces” (2010-2015)
- Advanced Low Carbon Technology Research and Development Program (ALCA)
- Next Generation Batteries “All-Solid-State Battery Team” Team leader (2013-2022)
No deterioration after 2,000 charges-discharges
The research team led by professor Masahiro Tatsumisago, the ALCA Research and Development team leader, developed a sulfide materials with the world's top-level conductivity as the electrode material for an “all-solid-state lithium/sulfur secondary battery,” which is anticipated to be the next-generation battery. They also succeeded in establishing the new electrode structure to maximize the use of sulfur with extremely high capacity density. This electrode combines a solid sulfide electrolyte with a lithium sulfide-based solid solution, and presents the highest capacity and lifespan among previously reported lithium-sulfur secondary batteries. The electrode that uses lithium sulfide-based solid solution has more than double the capacity of single lithium sulfide, does not deteriorate, and functions in a stable manner after 2,000 charges and discharges.
Among inorganic solid electrolytes, sulfide-based inorganic solid electrolytes have especially high ion conductivity; thus, their application to all-solid-state lithium batteries is anticipated. Conventionally, there have been problems with all-solid-state batteries in terms of low output and difficulty with operation under low temperatures. However, the ionic conductivity of sulfide-based solid electrolytes now exceeds that of the organic electrolyte solution used for lithium-ion batteries. This may lead to superior output and function under low temperatures compared to conventional all-solid-state batteries. As such, it is anticipated to become a power source for electric vehicles and households.
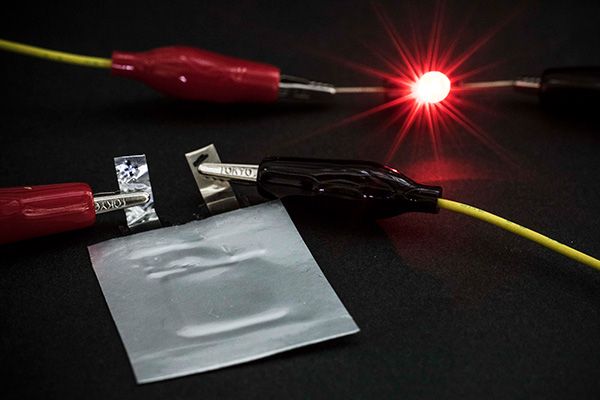
A prototype of sulfide-based all-solid-state battery.
Safety, lifespan, and low capacity as obstacles
Conventional lithium-ion batteries have a long lifespan and can be repeatedly charged. Though they have a high energy density, there have been issues in terms of safety such as accidental fires following heat generation since the electrolyte solution consist of a flammable organic solvent. Thus, in recent years, attention has shifted to all-solid-state batteries, in which organic solvent is replaced with a non-flammable inorganic solid electrolyte, as a next-generation power source.
To commercialize the lithium-sulfur secondary batteries, safety must be ensured by suppressing heat generation to prevent fires and allow for use across a wider range of temperatures. Higher energy density and a longer lifespan are also desired.
Lithium-sulfur secondary batteries that use organic electrolyte solution have a problem of deteriorating battery capacity, since lithium polysulfides, an intermediate reaction product, leaks into the electrolyte solution during electrode reaction. However, all-solid-state lithium-sulfur secondary batteries can fundamentally solve this problem. Since the lithium sulfide itself is an insulator, it is necessary to add conductivity to achieve higher energy density.
Various solutions have been examined in order to solve short battery lifespan and small capacity, but there had been few reports on lithium sulfide that retains high capacity even after 1,000 cycles or more of charging and discharging.
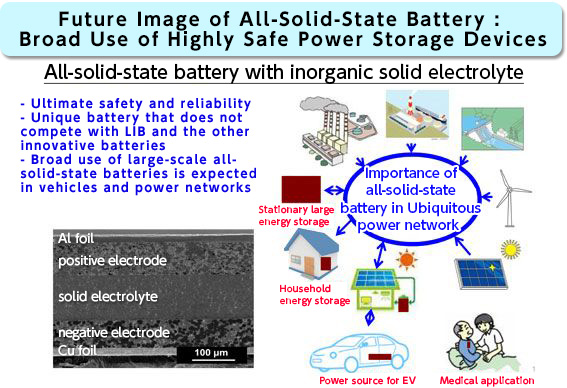
Solving the cause of heat generation using an electron microscope
Professor Tatsumisago created the world's best research team in terms of developing high ion conductivity materials and their material application technology, and first tackled the mechanism of heat generation reaction of electrodes, which is essential in terms of ensuring the safety of the batteries. The heat generation reaction is a cause of deterioration of batteries, and it is important to elucidate the mechanism of the heat generation in the battery materials. Therefore, the team collaborated with professor Shigeo Mori et al. to observe changes in microstructures and tissues of materials, and analyzed the thermal safety of materials.
When thermal behavior was examined while heating electrode materials and inorganic solid electrolytes, a heat generation reaction occurred at 300–400℃. To find the cause of this heat generation, the reaction was observed in real-time with an electron microscope. It showed that crystallization of the inorganic solid electrolytes where they come in contact with the electrode materials was the main cause of heat generation.
The next challenge is further clarification of the cause of the heat generation reaction including calculation of the crystallization energy, and analysis of the amorphous state. Professor Tatsumisago says he would like to “evaluate heat generation behavior of battery materials and its cause from various angles, and contribute toward practical implementation of all-solid-state lithium batteries.”
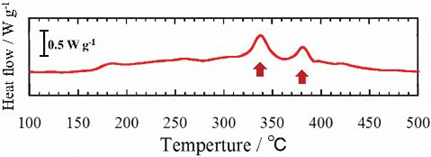
The result of differential scanning calorimetry analysis of the NMC-LPS cathode complex after charging.
There are two heat generation reactions within the temperature range of 300–400℃.
Toward doubling the energy density
In the future, the team aims to develop a thicker cathode layer, a thinner solid electrolyte layer, and high capacity anode materials, in order to increase the energy density of batteries. By combining these developments, the team aims to build all-solid-state lithium-sulfur secondary batteries with double the energy density of the conventional lithium-ion batteries. To accelerate such research, the team has begun collaborating with the Lithium Ion Battery Technology and Evaluation Center (LIBTEC).
In addition to sulfide-based inorganic solid electrolytes, the team studied oxide-based all-solid-state batteries. The team focused on improving the ionic conductivity of the solid-solid interface, and succeeded in realizing stable operation of oxide-based batteries at room temperature.
If safe and high-energy next generation batteries could be distributed throughout society, the performance and safety of automobiles and household electric appliances would drastically improve.
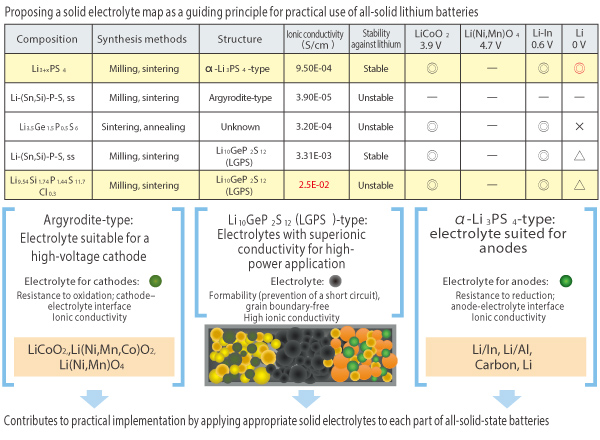
An example of material mapping for the sulfide-based solid electrolyte being created with Professor Ryoji Kanno et al.
- Environment and Energy
- Research Results
- Japanese
