|
1. New Methodology for Functionalization of Fullerenes
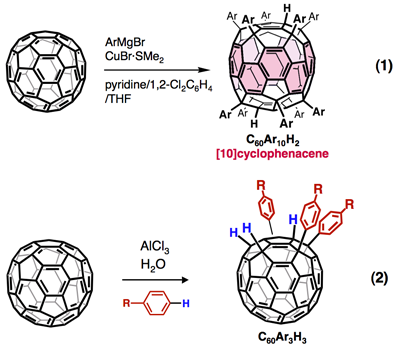
We are developing methods for the efficient synthesis of organic functionalized fullerene derivatives. Pyridine-modified organocopper reagents are used for the single-step deca-addition reactions to [60]fullerene to obtain deca(organo)[60]fullerenes, C60R10H2 (eq 1). Aluminum trichloride-mediated hydroarylation of [60]fullerene gives tri- and di-aryl adducts, C60Ar3H3 and C60Ar2H2, in an inexpensive manner using C60, arene, AlCl3, and H2O (eq 2).
Ref.
1. Y. Matsuo, E. Nakamura, Chem. Rev., 2008, 108, 3016-3028. [LINK]
2. Y. Matsuo, A. Muramatsu, K. Tahara, M. Koide, E. Nakamura, Org. Synth. 2006, 83, 80-87.
3. Y. Matsuo, K. Tahara, K. Morita, K. Matsuo, E. Nakamura, Angew. Chem. Int. Ed. 2007, 46, 2844-2847. [LINK]
4. A. Iwashita, Y. Matsuo, E. Nakamura, Angew. Chem. Int. Ed. 2007, 46, 3513-3516. [LINK]
5. Y. Matsuo, A. Iwashita, Y. Abe, C.-Z. Li, K. Matsuo, M. Hashiguchi, E. Nakamura, J. Am. Chem. Soc., 2008, 130, 15429-15436. [LINK]
2. Synthesis of Metal-Fullerene Complexes
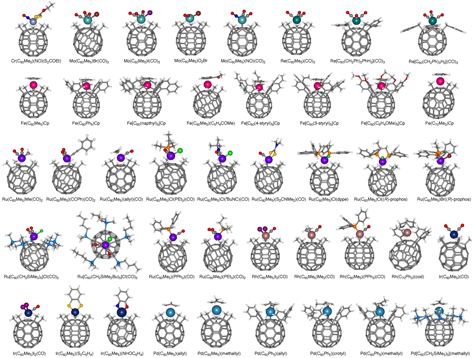
We are investigating the syntheses, structures, and properties of metal-fullerene complexes. The main area of interest to the group is the construction of donor-acceptor systems using electron-donating transition metal complexes and electron-accepting fullerene parts. Half-metallocene complexes of Group 6-10 metals (Cr, Mo, W, Re, Mn, Fe, Ru, Co, Rh, Ir, Ni, Pd, Pt, etc.) are useful materials for organometallic chemistry of the metal-fullerene complexes.
Ref.
1. M. Sawamura, Y. Kuninobu, M. Toganoh, Y. Matsuo, M. Yamanaka, E. Nakamura, J. Am. Chem. Soc. 2002, 124, 9354-9355. [LINK]
2. M. Toganoh, Y. Matsuo, E. Nakamura, J. Am. Chem. Soc. 2003, 125, 13974-13975. [LINK]
3. Y. Matsuo, E. Nakamura, J. Am. Chem. Soc. 2005, 127, 8457-8466. [LINK]
4. Y. Matsuo, H. Isobe, T. Tanaka, Y. Murata, M. Murata, K. Komatsu, E. Nakamura, J. Am. Chem. Soc. 2005, 127, 17148-17149. [LINK]
3. Electrochemical and Photophysical Functions
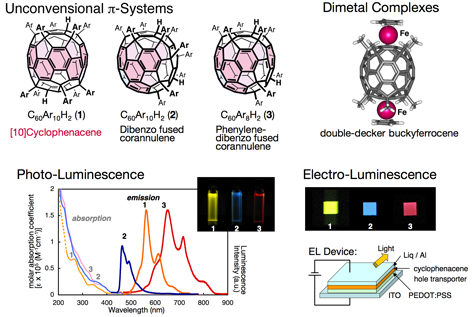
We are exploring new electrochemical and photophysical functions of functionalized fullerenes and fullerene metal complexes. Deca(organo)[60]fullerenes, C60R10H2, have hoop-shaped cyclic pi-electron conjugated systems, so-called [10]cyclophenacenes, and exhibit unique photo-luminescence and electro-luminescence properties. Various di-metal complexes, for instance double-decker buckyferrocenes (CpFe)2(C60R10) are employed to discuss electronic interaction between two transition metal atoms through the fullerene pi-system. Metal-fullerene complexes show ultra-fast photoinduced charge separation behavior because of the direct connection of the metal atoms and the fullerene cage.
Ref.
1. E. Nakamura, K. Tahara, Y. Matsuo, M. Sawamura, J. Am. Chem. Soc. 2003, 125, 2834-2835. [LINK]
2. Y. Matsuo, K. Tahara, M. Sawamura, E. Nakamura, J. Am. Chem. Soc. 2004, 126, 8725-8734. [LINK]
3. Y. Matsuo, K. Tahara, E. Nakamura, J. Am. Chem. Soc. 2006, 128, 7154-7155. [LINK]
4. Y. Matsuo, Bull Chem. Soc. Jpn., 2008, 81, 320-330. (Award Accounts). [LINK]
4. Liquid Crystalline and Supramolecular Functions
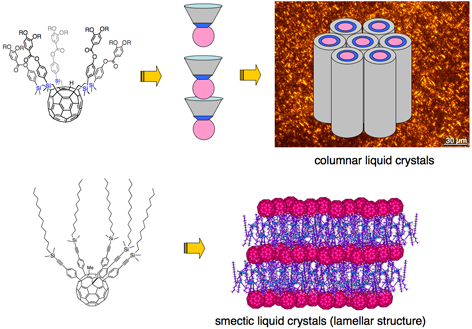
Fullerene-containing liquid crystals are promising electro/photo active soft materials. However, the size and shape of the C60 unit tends to disturb ordered liquid crystalline structures. We are synthesizing shuttlecock-shaped fullerene liquid crystalline molecules, in which the C60 unit represents a core for the supramolecular ordered columnar organization. On the other hand, bifunctional molecules containing aromatic fullerene and aliphatic alkyl chains form lamellar liquid crystalline structure.
Ref.
1. M. Sawamura, K. Kawai, Y. Matsuo, K. Kanie, T. Kato, E. Nakamura, Nature 2002, 419, 702-705. [PDF]
2. Y. Matsuo, A. Muramatsu, R. Hamasaki, N. Mizoshita, T. Kato, E. Nakamura, J. Am. Chem. Soc. 2004, 126, 432-433. [LINK]
3. Y. Matsuo, A. Muramatsu, Y. Kamikawa, T. Kato, E. Nakamura, J. Am. Chem. Soc. 2006, 128, 9586-9587.
4. Y.-W. Zhong, Y. Matsuo, E. Nakamura, J. Am. Chem. Soc. 2007, 129, 3052-3053. [LINK]
5. Photo-Current Conversion Functions
Conversion of solar energy to electric energy is one of the most important issues in this century. We are synthesizing lunar-landing-module shaped rigid and compact photocurrent generating molecules containing ferrocene donor, fullerene acceptor, and five carboxylic acid groups to fix the molecules onto electrode surfaces. We have so far succeeded in switching photocurrent direction by changing excited states (i.e., triplet or charge separatation) with the choice of molecular component (organic or organometallic) as well as by changing molecular orientation. Such well-defined photo-electronic functions is expected to be useful in organic photovoltaics and information-carrying molecular devices.
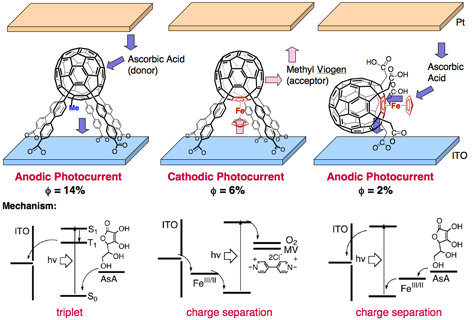
Ref.
1. Y. Matsuo, K. Kanaizuka, K. Matsuo, Y.-W. Zhong, T. Nakae, E. Nakamura, J. Am. Chem. Soc. 2008, 130, 5016-5017.[LINK]
Research Stuff and Students
Dr. Yutaka Matsuo (Leader)
Dr. Aiko Sakamoto (Researcher, JST)
Dr. Chang-Zhi Li (Researcher, JST)
Dr. Zuo Xiao (Researcher, JST)
Dr. Keiko Matsuo (Researcher, JSPS)
Takeshi Fujita (PhD students)
Takahiko Ichiki (PhD students)
Ying Zhang (PhD student)
Sebastian Lacher (PhD student)
Masashi Maruyama (Master course)
Hiromi Oyama (Master course)
Katsumasa Nakahara (Undergraduate)
|





