
Research
Glycosylation is one of the most important and extensive post-translational modifications of proteins. Structure of glycan chains on eukaryote glycoproteins is quite heterogeneous, and their biological roles are correspondingly diverse. For example, it is well accepted that glycan chains are involved in the stability and solubility of proteins, cell-cell recognition, cell differentiation, carcinogenesis, signal transduction, immune response, infection of microorganisms, and so on. Glycan chains of glycoproteins are classified into asparagine-linked (N-linked) type, serine/threonine-linked (O-linked) type, and the other minor types. Their structural diversity is called “glycoform”, which arises from differences in the number, composition, sequence, branching, and linkage position of sugar residues. These biochemical properties enable glycans to carry large biological information.
However, in comparison with polypeptide and polynucleotides, methodology for glycan chains to read and reconstitute their intrinsic structural information has been insufficient. Methods for synthesizing peptides and oligonucleotides are well established and can be easily performed using automated synthesizers. In contrast, chemical synthesis of glycan chains is far less straightforward and requires highly complicated maneuvers, because most of them have branched structures, and stereochemical isomerisom derived from anomeric linkages must be strictly controlled. So far, automatic synthesis of some specific glycans has been reported, its practicality has been severely limited. Under these circumstances, precise biochemical experiments with homogeneous glycan chains are extremely difficult, preventing researchers in other fields enlisting in functional studies of glycoproteins.
Aim of our project is to perform molecular level researches for glycoconjugates by extensively exploiting the capacity of organic chemistry. Our study will explore facile methods for chemical synthesis of pure glycan chains and functionalized derivatives, which will be utilized for various biochemical and biological experiments. Using these resources will allow us to clarify biological roles of glycan chains in a clear-cut fashion. This strategy will provide break-through in glycobiology or ‘glycoprotein’ biology, across multiple areas ranging from basic science to medical, pharmaceutical, and agricultural applications.
GLYCOTRILOGY project, consisting of three research groups, aims to achieve synergic development of ‘trinal’ areas, including synthetic organic chemistry, analytical chemistry, and molecular biology, in order to explore and find novel aspects of glycobiology. The ‘Biofunctional glycochemistry’ group explores facile and versatile methods for the synthesis of various glycoconjugates, and utilize physicochemical methods to analyze carbohydrate-protein interactions, which have become one of the major subjects in post-genome era. The ‘Chemical glycoinformation’ group explores innovative methods for analysis of glycan structure in sub-picomole, tertiary structure of glycoproteins, and metabolic system of glycoconjugates in living cells. The ‘Synthetic glycoprotein’ group establishes general methods for chemical synthesis of homogeneous glycoproteins, which will serve to produce glycoprotein medicines in near future. We predict that our strategies and efforts will bring sizable contributions in basic as well as in applied glycosciences.
Biofunctional Glycochemistry
We chemically synthesize high mannose-type glycans and their derivatives,
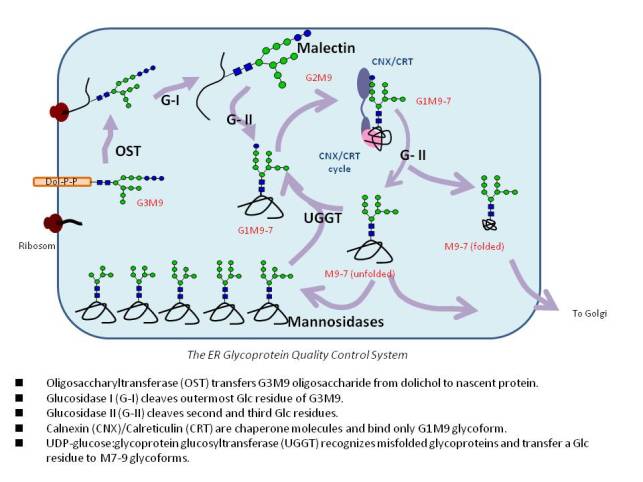
and using them, we studies the intracellular functions of N-linked glycans in the quality control system in the ER.
Glycans linked to proteins and lipids regulate their biochemical properties.
Recently, it has become clear that glycans serve as not only hydrophilic
moieties but also informative molecules. In most cases, molecules receiving
glycan-specific signals are proteins. Elucidation of the molecular mechanism
of interaction between glycans and proteins will lead to better understanding
of biological phenomena. However, the greatest hindrance in the analysis
of glycan–protein recognition has been difficult for large-scale preparation
of defined and homogeneous oligosaccharides that can be subjected to various
analyses. We will address the large-scale synthesis of high-mannose-type
oligosaccharides and analyze carbohydrate–protein interactions.
Synthetic Glycoprotein
We chemically synthesize glycoproteins bearing human-type N-linked glycans, and study the effects of glycans on protein function through
chemical approach. These studies will lead to the elucidation of glycan
functions and the exploration of glycoprotein medicines.
Synthesis of homogeneous glycoproteins bearing high-mannose or complex
type biantennary oligosaccharides
-------------------------------------------------------------------------------------------------------------------------------
Protein glycosylation is one of the most important post-translational modification on protein surface and the presence of carbohydrates on proteins is associated with a number of biological events. In nature glycoproteins are found in complex heterogeneous mixture, which complicates analyses of their characterisation and function. For understanding the role of each carbohydrate substituent on the glycoprotein, it is therefore essential to have access to homogeneous glycoproteins. Using small model protein we are going to synthesize homogeneous glycoproteins bearing high-mannose type or asialo complex type oligosaccharides in order to investigate the functions of N-glycans on the protein structure. In order to prepare homogeneous glycoproteins we used both peptide solid phase synthesis and Native Chemical Ligation (NCL). The full length amino acid sequence is divided into several segments that have been prepared using Fmoc or Boc chemistry and later on are combined using NCL. With this methodology we prepared both homogeneously folded and misfolded proteins bearing high mannose type oligosaccharides.
In general, folded proteins exhibit a hydrophilic nature, while misfolded proteins show more hydrophobic behavior. (UDP)-Glucose: glycoprotein glucosyltransferase (UGGT) is an ER-resident enzyme which recognizes misfolded glycoproteins and is responsible for transfering a glucose unit to the specific nonreducing terminal of high-mannose glycans of misfolded glycoproteins. Recent studies on UGGT show that this enzyme recognizes subtle acceptor substrate such as methotrexate-M9 (MTX-M9), where the MTX is apparently mimicking the hydrophobic property of misfolded proteins. Therefore homogeneously misfolded glycoproteins bearing high-mannose type oligosaccharides will be useful probes to investigate the complex process governing glycoprotein folding in the endoplasmic reticulum.
Chemical Glyco-information
We will analyze tertiary structures of glycoproteins and biosynthetic process
of glycosphingolipids in the Golge apparatus, by means of organic chemistry,
analytical chemistory and biochemistry.
Elucidating a complex glycan structure is the subject of prime importance.
Investigation of the individual structure of glycoforms presented at different
positions of glycoproteins is the current focal area. Amounts and compositions
of glycans on the plasma membrane presented as glycoproteins and glycolipids
fluctuate spatially and temporally depending on an environment of the cell.
No method has been provided to address spatio-temporal, structural, and
quantitative issues. In the meanwhile, although structural information
of proteins is being accumulated and available in the drag-design, a majority
of proteins is glycosylated and awaits to be solved. The group will make
efforts to fulfill the missing link in glycobiology based on chemistry,
analytical chemistry, and cell biology.