- JST Home
- /
- Strategic Basic Research Programs
- /
 ERATO
ERATO- /
- Research Area/Projects/
- Completed/
- TAKAI Biotimer
TAKAI Biotimer

Research Director: Dr. Yoshimi Takai
(Professor, Graduate School of Medicine, Osaka University)
Research Term: 1994-1999
The cells of multicellular organisms accomplish their roles with exquisitely appropriate timing, even while experiencing a wide variety of extracellular stimuli. After defining this timing/switching system as biotimer, its molecular mechanisms involving memory formation at synaptic junctions in the brain, as well as cell migration, proliferation, and adhesion in the epithelial cells, were investigated.
Research Results
Discovery of time-controlling regulators of neurotransmitter release and clarification of their functions: An inhibitory regulator for the Rab family members (Rab GDI) was found to participate in time control of neurotransmitter release. A novel stimulatory regulator for Rab3 small G protein (Rab3 GEP) was found, and it was elucidated that Rab3 GEP also participates in neurotransmitter release and memory formation.
Clarification of functions of small G protein regulators in ontogeny: Knock-out mice were produced for two regulators discovered in our laboratory: Rho GDI, a regulator for the Rho family members that participate in the cytoskeletal control, and Smg GDS, a regulator for Ki-Ras and the Rap and Rho family members. Ki-Ras and the Rap family members participate in the control of cell proliferation and differentiation. It was then possible to elucidate a part that these regulators play in time control of ontogeny.
Discovery of a time-controlling regulator of cell migration and axonal extension and clarification of its function: A new stimulatory regulator for Cdc42 small G protein (frabin) was found to regulate changes in the actin cytoskeleton, which is important for cell morphology, cell migration, and axonal extension. Frabin stimulates Cdc42, which controls the actin cytoskeleton structure, depending on a preexisting actin cytoskeleton, and induces new actin cytoskeleton (filopodia) formation.
Discovery of new F-actin-binding proteins for cell-cell and cell-matrix junctions: Two new F-actin-binding proteins (neurabin and nexilin) were found. Neurabin controls the actin cytoskeleton (lamellipodia) formation, and then participates in the regulation of axonal extension. Nexilin is involved in the connection between cell-matrix junctions and the actin cytoskeleton.
Discovery of a new cell-cell adhesion system and explanation of its function: A new cell-cell adhesion system at adherens junctions was found, which has been named the NAP system. It consists of at least three components: nectin, afadin, and ponsin. Nectin is a cell adhesion molecule and afadin connects nectin to the actin cytoskeleton. Ponsin links nectin and afadin to the cadherin system, which is a well-known cell-cell adhesion system. The NAP system is implicated in the formation of adherens and tight junctions.
Discovery of new components of synaptic junctions: Many new components of synaptic junctions (SAPAP, S-SCAM, BEGAIN, MAGUIN, synamon, nRapGEP, nArgBP2) were found, resulting in a proposal of how the receptors and adhesion molecules are assembled in the same location, the synapses, and participate in the deposition of memory.
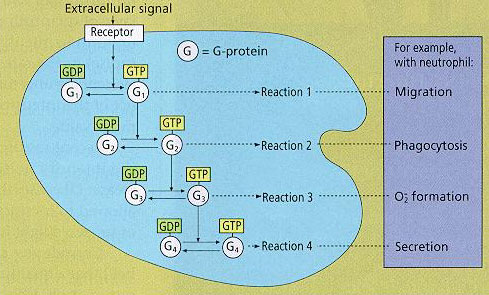
Concept of Biotimer
G-proteins determine the duration of the reactions they control and, at the same time, they also regulate the order in terms of time of reactions 1, 2, 3 and 4 so that these occur in this sequence following a single extracellular signal.

















