- JST Home
- /
- Strategic Basic Research Programs
- /
 ERATO
ERATO- /
- Research Area/Projects/
- Completed/
- KATO Cytoprotein Network
KATO Cytoprotein Network

Research Director: Dr. Seishi Kato
(Chief Researcher, Sagami Chemical Research Center)
Research Term: 1995-2000
The main purpose of this project was to identify novel intracellular protein (cytoprotein) networks using an approach based on the full-length cDNA bank.
Research Results
Discovery of a NEDD8 modification system regulating the cell cycle: During in vitro translation analysis of full-length cDNA clones, a ubiquitin-like protein, NEDD8, was found to covalently modify cullin-family proteins; three proteins composed of this pathway were identified. Using a fission yeast system, NEDD8 was shown to regulate the cell cycle via the modification of an SCF (Skp 1/cullin-1/F-box protein) complex. These results indicate that NEDD8 modification plays an essential role in various regulation processes involving cullins.
Discovery of a novel nuclear protein complex with interaction via the WW domain: A novel nuclear protein containing the WW domain, Npw38, was identified in the full-length cDNA bank. A search for Npw38-interacting molecules revealed that Npw38 can interact with NpwBP, DNA helicase, RNA helicase, RNA, and single-stranded DNA to form a nuclear protein complex which may be involved in transcriptional events as a member of an mRNA factory.
Discovery of a novel O-glycosylation of intracellular proteins: Rabbit muscle cytosolic creatine kinase was found to be O-glucosylated via Ser and Thr residues. Other intracellular proteins, such as metabolic enzymes, were shown to contain sugar molecules. These results imply the existence of a novel glycosylation system in the cytosol, by which some cellular function might be regulated.
Localization to function: A comprehensive localization analysis of novel full-length cDNA clones was carried out using fusion genes with urokinase cDNA for membrane proteins or green fluorescent protein cDNA for intracellular proteins, while identifying many novel proteins, including type II membrane proteins, a spliceosome component, as well as RNA helicase, and immortalization-upregulated nuclear proteins.
Antibody production using cDNA immunization: When an expression vector carrying human full-length cDNA was inoculated into a mouse or rat using a syringe or gene gun, antibody production against the protein encoded by each cDNA was observed in the serum. Furthermore, a method to improve the efficiency of antibody production was developed.
Two-hybrid localization method for protein-protein interaction analysis: A novel method to rapidly and visually detect a protein-protein interaction occurring under physiological conditions in a cell was developed, based on an observation of the localization change of a reporter-fused protein induced by an interaction with a protein possessing a localization signal. This method enables one to perform a comprehensive analysis of protein-protein interactions among proteins encoded by the full-length cDNA clones.
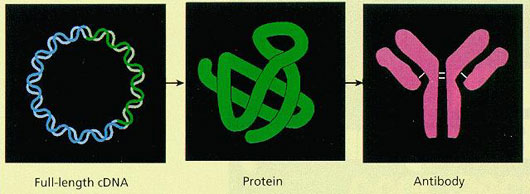
A Toolbox
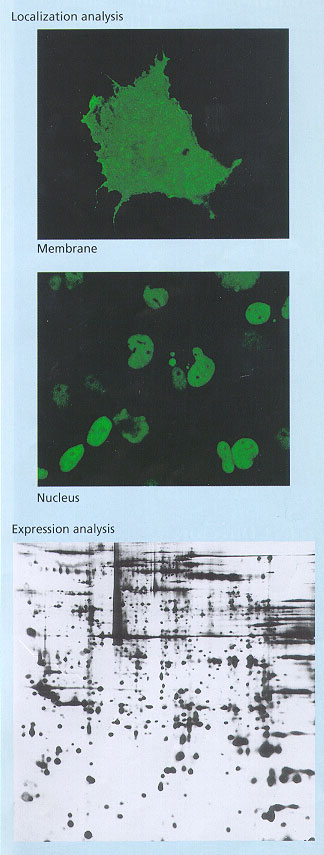
Examples of cytoprotein network analysis

















