- JST Home
- /
- Strategic Basic Research Programs
- /
 ERATO
ERATO- /
- Research Area/Projects/
- Completed/
- INOUE Photo-chirogenesis
INOUE Photo-chirogenesis

Research Director: Dr. Yoshihisa Inoue
(Professor, Graduate School of Engineering, Osaka University)
Research Term: 1996-2001
Since essentially all syntheses of optically active compounds currently use optically pure catalysts or starting materials, chemists are dependent upon nature for their supply. New knowledge for making chiral compounds is thus needed to overcome this dependency and to open new routes for synthesizing many interesting and important new chiral compounds. The Inoue Photochirogenesis project used powerful new tools and innovative new approaches in a challenge to a centuryold dream of chemists: to use light to create asymmetric molecules.
Reseaerch Results
New methods for absolute asymmetric synthesis using circularly polarized light: The norbornadiene-quadricyclane system has been made to undergo a clean photo-reversible reaction. Simultaneous enantiomeric enrichments in both the reactant and the product were achieved for the first time. In the same molecular system, two-photon CPL excitation was demonstrated to enhance the efficiency of enantiomeric enrichment, utilizing the anisotropy factor (g*) of the excited state.
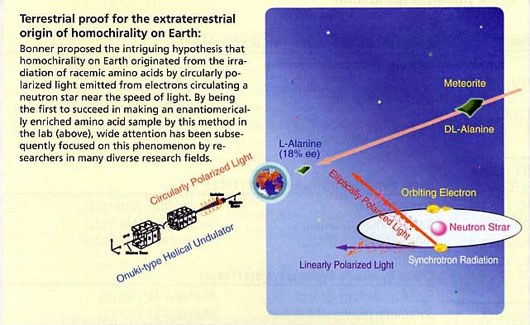
New Evidence Supporting the Bonner hypothesis: Bonner proposed the intriguing hypothesis that homochirality on Earth originated from the irradiation of racemic amino acids by CPL emitted from electrons circulating a neutron star. By being the first to succeed in making an enantiomerically enriched amino acid sample by this method in the lab, wide attention has been focused on this phenomenon.
Environmental multidimensional control of photoproduct chirality: By regulating environmental factors, such as the temperature, pressure and solvent, it was possible to selectively obtain both R- and S-types of a photoproduct from the same starting material. This control has been interpreted in terms of the contributions of both enthalpy and entropy. A new concept has thus been proposed: “entropic multidimensional control of chirality.”
Achievement of the world-best ee for photosensitized asymmetric synthesis: By exploiting the entropic multidimensional control of chirality experimentally, a world-first enantiomeric excess of 100% was reached for a photosensitized unimolecular reaction, and 40% for a bimolecular photosensitization, far overwhelming the previous record (6.7%).
Chiral transition metal complexes as novel photosensitizers: The Δ/Λ chirality of octahedral metal complex was successfully controlled by inter-ligand interactions, yielding chiral ruthenium complexes for novel asymmetric photocatalysis.
Chiral environments for supramolecular photoreactions: Supramolecular photochirogenesis is a completely new area of chemistry. Native and modified cyclodextrins have been employed as chiral hosts to photochemically induce chirality in included guest molecules in the world-best enantiomeric excess of 41% (the previous record was 15% ).
Supramolecular chirality sensor: A metal-containing porphyrin dimer was synthesized. This agent directly determines the absolute configuration of chiral amines and alcohols without any additional modification. Its industrial application is now underway.
Thermodynamic concepts that direct both chiral interactions and molecular recognition in chemistry and biology: For the first time, the process of chiral recognition was systematically analyzed thermodynamically, revealing a global compensatory enthalpy-entropy relation-ship.
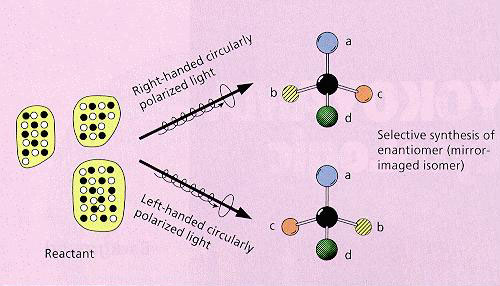
·Schematic drawing of the absolute asymmetric synthesis
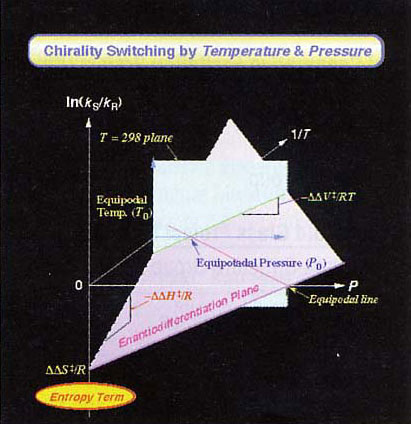
Representative 3-D Temperature-Pressure-Enantioselectiviity Diagram Established for Asymmetry Photosensitization

















